Why Are Realistic Aneurysm Models Crucial for Device Evaluation?
Anatomical Accuracy and Physiological Fidelity
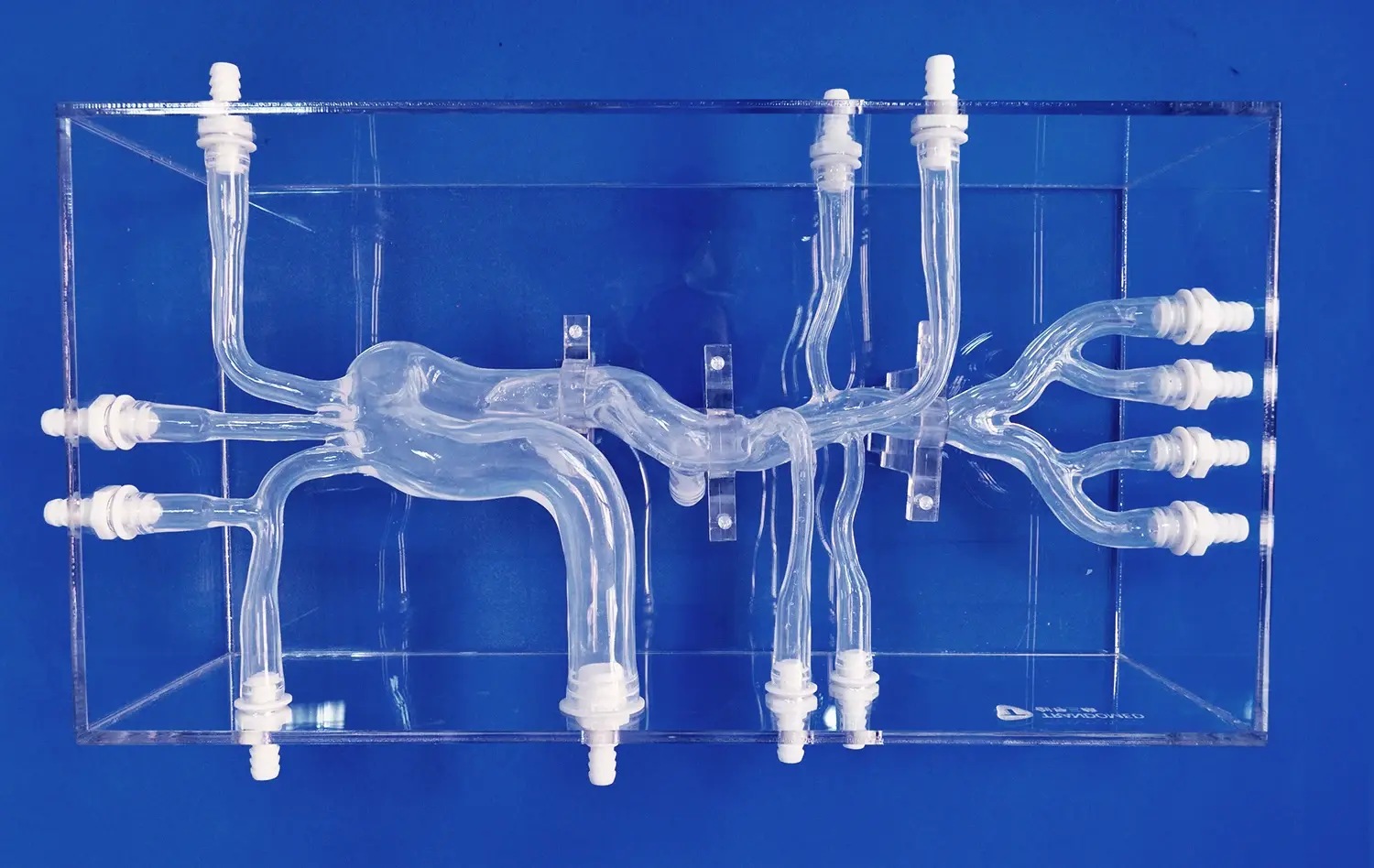
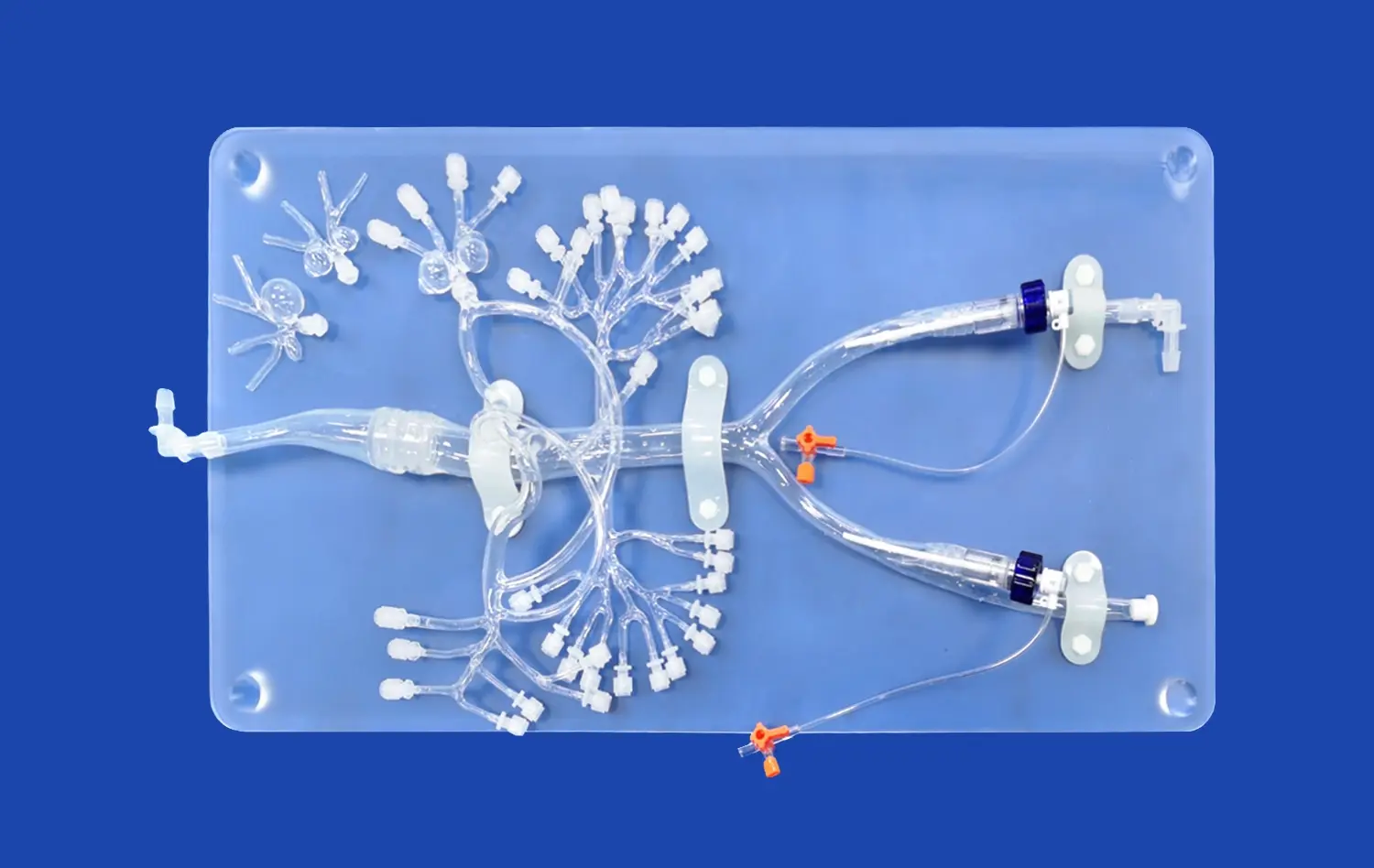
Realistic aneurysm models are indispensable for device evaluation due to their unparalleled anatomical accuracy. These models, such as the SJX011 Intracranial Vascular with Aneurysm Model, meticulously replicate the intricate network of cerebral blood vessels, including the complex geometry of aneurysms. This level of detail allows researchers and clinicians to assess how neurovascular devices interact with vessel walls, navigate tortuous pathways, and deploy within aneurysm sacs under conditions that closely mimic in vivo scenarios.
Moreover, advanced aneurysm models can simulate physiological conditions like blood flow and vessel wall compliance. This physiological fidelity is crucial for evaluating the performance of flow diverters, coils, and other endovascular devices. By replicating the pulsatile nature of blood flow and the elastic properties of vessel walls, these models provide invaluable insights into how devices will behave in real-world clinical situations.
Risk-Free Experimentation and Iteration
One of the primary advantages of using aneurysm models for device evaluation is the ability to conduct risk-free experimentation. Unlike clinical trials, which involve inherent risks to patients, these models allow for repeated testing and refinement of devices and techniques without ethical concerns. This iterative process is essential for identifying potential issues, optimizing device design, and improving deployment strategies before human trials begin.
Researchers can manipulate variables such as aneurysm morphology, parent vessel geometry, and flow conditions to assess device performance across a spectrum of challenging scenarios. This comprehensive evaluation helps in predicting potential complications and developing mitigation strategies, ultimately enhancing the safety and efficacy of neurovascular interventions.
Standardization and Reproducibility
Aneurysm models facilitate standardization in device evaluation, a critical factor in the regulatory approval process. By using consistent, well-characterized models, researchers can generate reproducible results that are essential for validating device performance. This standardization allows for meaningful comparisons between different devices and techniques, providing a solid foundation for evidence-based decision-making in clinical practice.
Furthermore, the ability to create multiple identical models enables multi-center studies and collaborative research efforts. This reproducibility accelerates the pace of innovation in neurovascular medicine by allowing simultaneous evaluations across different institutions, fostering knowledge sharing, and expediting the development of consensus guidelines for new technologies.
Performance Assessment of Neurovascular Implants in Simulated Aneurysm Environments
Deployment and Positioning Accuracy
Assessing the deployment and positioning accuracy of neurovascular implants is a critical aspect of their performance evaluation. Aneurysm models provide an ideal platform for this assessment, allowing researchers to observe and measure the precision with which devices such as stents, flow diverters, and embolic coils can be placed within the simulated vascular structures.
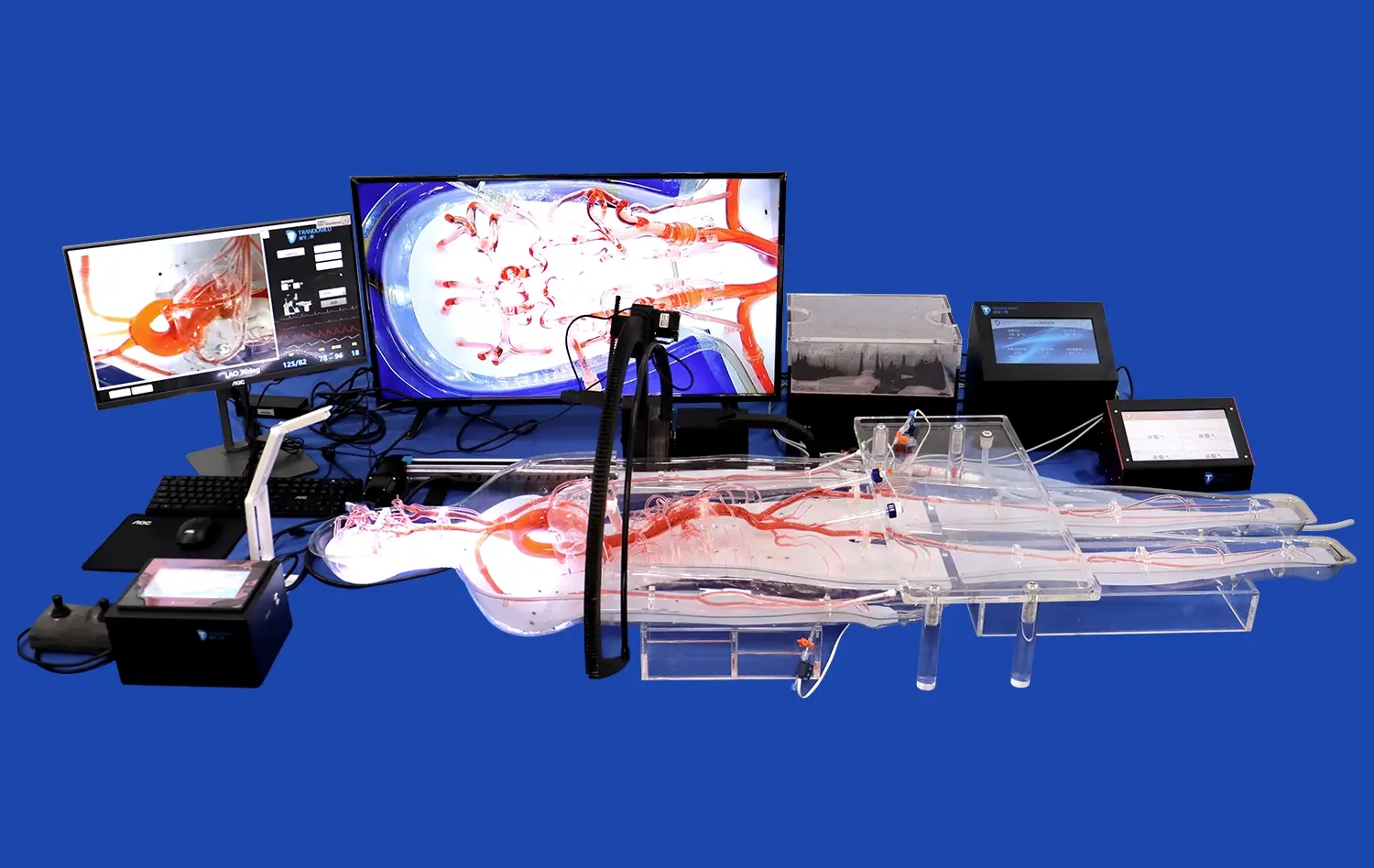
Using transparent or semi-transparent materials in model construction enables real-time visualization of device deployment. This visibility is particularly valuable for evaluating the navigation of microcatheters through tortuous vessels and the final positioning of implants within aneurysm sacs or parent arteries. Researchers can analyze factors such as radial force, conformability to vessel walls, and the ability to maintain desired positions under simulated physiological conditions.
Flow Dynamics and Aneurysm Occlusion
One of the primary goals of neurovascular implants is to alter flow dynamics within aneurysms to promote thrombosis and eventual occlusion. Advanced aneurysm models equipped with flow simulation capabilities allow for detailed analysis of how different devices affect blood flow patterns. By incorporating fluid dynamics principles, these models can demonstrate the effectiveness of flow diverters in reducing aneurysm inflow and the impact of coil packing density on stagnation zones.
Researchers can employ various imaging techniques, such as particle image velocimetry or computational fluid dynamics, to quantify changes in flow velocities, wall shear stress, and residence times within the aneurysm sac. This data is crucial for predicting the likelihood of successful aneurysm occlusion and assessing the risk of post-treatment complications like coil compaction or aneurysm recurrence.
Long-term Performance and Durability
While acute performance is important, the long-term efficacy and durability of neurovascular implants are equally critical. Aneurysm models designed for extended testing periods can provide valuable insights into device behavior over time. These models can be subjected to accelerated aging protocols that simulate years of physiological stress in a condensed timeframe.
Researchers can evaluate factors such as material fatigue, corrosion resistance, and the potential for device migration or fracture. Additionally, models that incorporate bioactive materials can help assess the tissue response to implants, including the rate of endothelialization or the risk of in-stent stenosis. This long-term performance data is essential for predicting the clinical outcomes of neurovascular interventions and guiding improvements in device design and materials.
How Do Aneurysm Models Improve Clinical Translation of Endovascular Innovations?
Bridging the Gap Between Bench and Bedside
Aneurysm models play a pivotal role in bridging the gap between laboratory research and clinical application of endovascular innovations. These sophisticated replicas serve as an intermediary step, allowing for thorough evaluation and refinement of new techniques and devices before they reach human trials. By closely mimicking the anatomical and physiological conditions encountered in real patients, aneurysm models provide a controlled environment where researchers can identify potential challenges and optimize solutions.
The ability to test innovations on a wide range of aneurysm morphologies and vessel configurations helps in predicting their performance across diverse patient populations. This comprehensive pre-clinical assessment significantly reduces the risks associated with first-in-human trials and accelerates the path to clinical adoption. Moreover, the insights gained from model-based testing often inform the design of clinical trials, ensuring that the most critical aspects of device performance are thoroughly evaluated.
Enhancing Physician Training and Procedural Planning
Aneurysm models are invaluable tools for enhancing physician training and procedural planning. These models allow interventional neuroradiologists and neurosurgeons to practice new techniques and familiarize themselves with novel devices in a risk-free environment. The tactile feedback and visual cues provided by high-fidelity models, such as those offered by Trandomed, closely replicate the challenges encountered during actual endovascular procedures.
For patient-specific planning, 3D-printed aneurysm models based on individual imaging data can be created. These personalized models enable surgeons to rehearse complex procedures, select the most appropriate devices, and anticipate potential complications before entering the operating room. This level of preparation not only improves procedural outcomes but also enhances patient safety by reducing intraoperative decision-making and shortening procedure times.
Facilitating Regulatory Approval and Clinical Adoption
The use of standardized aneurysm models in the evaluation of endovascular innovations greatly facilitates the regulatory approval process. Regulatory bodies increasingly recognize the value of data obtained from high-fidelity simulations as part of the evidence package for new device submissions. The ability to demonstrate safety and efficacy in a controlled, reproducible environment can expedite the approval timeline and reduce the need for extensive animal studies.
Furthermore, the data generated from aneurysm model testing can be instrumental in gaining clinical acceptance of new technologies. By providing clear, quantifiable evidence of device performance across various anatomical and physiological conditions, these models help build confidence among clinicians. This evidence-based approach to innovation translation not only accelerates the adoption of promising new treatments but also ensures that patients receive the most effective and safe interventions available.
Conclusion
Aneurysm models have emerged as indispensable tools in the realm of neurovascular medicine, offering a safe and effective means of testing innovative techniques and devices. These sophisticated replicas provide researchers and clinicians with invaluable insights into device performance, flow dynamics, and long-term efficacy. By bridging the gap between laboratory research and clinical application, enhancing physician training, and facilitating regulatory approval, aneurysm models are driving rapid advancements in the field. As we continue to push the boundaries of endovascular innovation, the role of these models in improving patient outcomes and revolutionizing aneurysm treatment cannot be overstated.
Contact Us
For more information on cutting-edge aneurysm models and how they can benefit your research or clinical practice, contact Trandomed. Our team of experts is ready to assist you in finding the perfect solution for your neurovascular simulation needs. Reach out to us at jackson.chen@trandomed.com to explore our range of customizable, high-fidelity aneurysm models and take the next step in advancing neurovascular care.
_1736216292718.webp)
_1734507815464.webp)
1_1732869849284.webp)