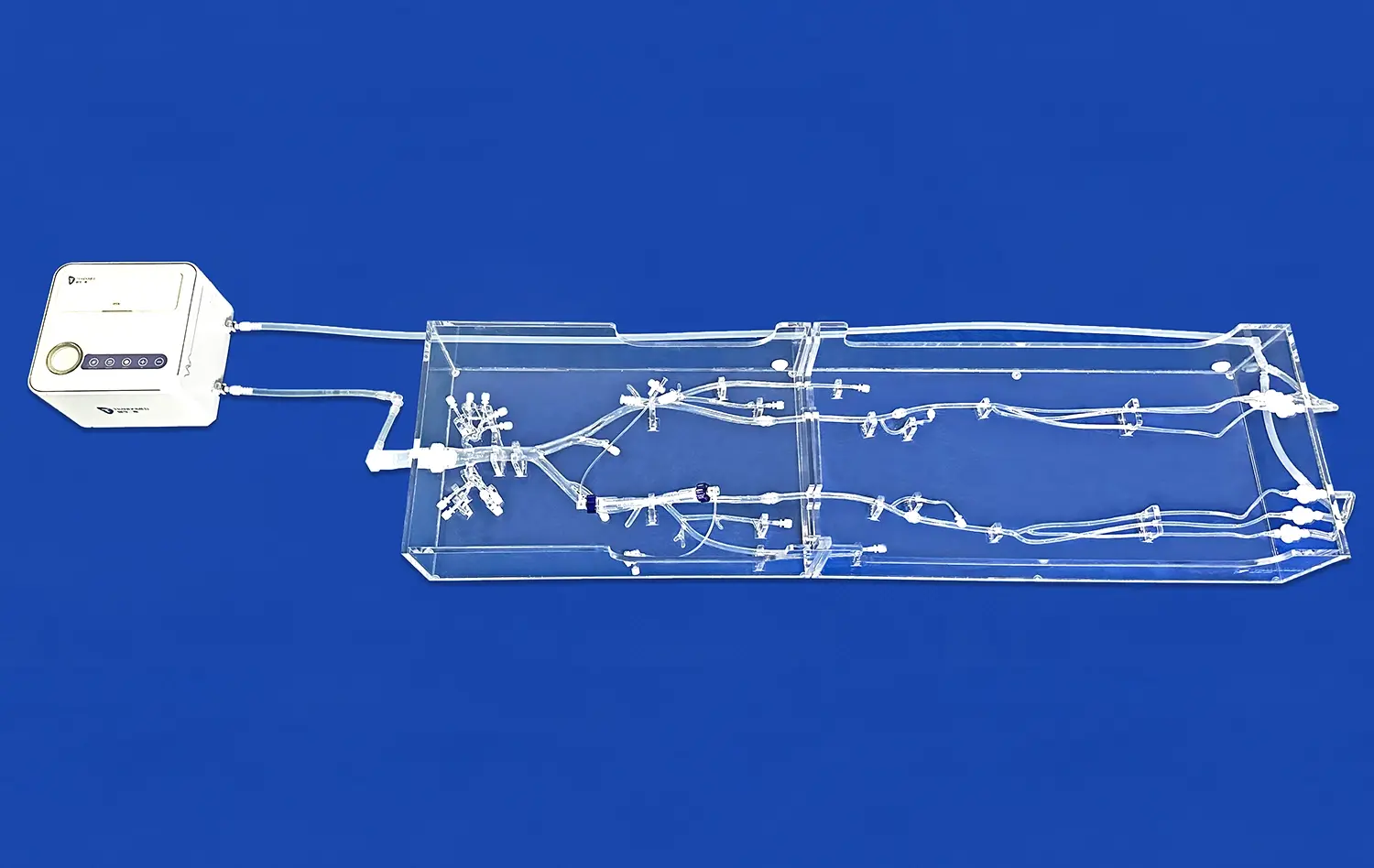
Why Are Aneurysm Models Ideal for Preclinical Evaluation?
Anatomical Accuracy and Variability
Aneurysm models, such as the SJX011 Intracranial Vascular with Aneurysm Model, offer unprecedented anatomical accuracy. These models meticulously replicate the complex cerebral vasculature, including the anterior cerebral artery (ACA), middle cerebral artery (MCA), and the tortuous internal carotid artery. The inclusion of multiple aneurysms at various locations allows researchers to study different anatomical scenarios, crucial for comprehensive device evaluation.
Customization Capabilities
The ability to customize aneurysm models is a game-changer in preclinical evaluation. Manufacturers like Trando 3D Medical Technology Co., Ltd. offer extensive customization options. Researchers can specify the number, size, and location of aneurysms, as well as adjust the tortuosity of arteries to reflect diverse clinical situations. This flexibility enables the creation of patient-specific models, allowing for targeted testing of devices in unique anatomical configurations.
Material Properties and Physiological Simulation
Advanced aneurysm models are crafted from materials that closely mimic the properties of human blood vessels. This realism extends to the elasticity and compliance of the vessel walls, crucial factors in device deployment and performance. Some models even incorporate pulsatile flow simulation, providing a dynamic environment that closely resembles in vivo conditions. This level of physiological simulation is essential for accurately predicting device behavior and potential complications.
Simulated Environments for Stent and Catheter Testing
Navigational Challenges and Device Delivery
Aneurysm models provide an ideal platform for testing the navigability of catheters and stents through complex vascular structures. The tortuous pathways and varying diameters of cerebral arteries present significant challenges in device delivery. By using these models, engineers can assess the trackability, pushability, and overall maneuverability of their devices in a controlled, yet realistic environment. This testing is crucial for refining device designs to ensure smooth and accurate deployment in clinical settings.
Deployment and Positioning Accuracy
The precise deployment of stents and coils is critical in aneurysm treatment. Aneurysm models allow for detailed evaluation of deployment mechanisms and positioning accuracy. Researchers can observe how different devices interact with the simulated vessel walls and aneurysm sacs, assessing factors such as wall apposition, coverage of the aneurysm neck, and potential for device migration. This level of detail in testing helps in optimizing device designs for improved clinical outcomes.
Flow Diversion and Hemodynamic Effects
Advanced aneurysm models can be integrated with flow simulation systems to study the hemodynamic effects of various devices. This capability is particularly valuable in assessing flow-diverting stents and their impact on aneurysm hemodynamics. Researchers can visualize and quantify changes in blood flow patterns, stagnation zones, and wall shear stress - all critical factors in predicting the effectiveness of aneurysm treatment and the potential for complications such as thrombosis or recanalization.
Accelerating Innovation in Endovascular Device Development
Rapid Prototyping and Iteration
Aneurysm models significantly accelerate the innovation cycle in endovascular device development. By providing a platform for rapid prototyping and testing, these models allow engineers to quickly iterate on device designs. This accelerated process enables faster refinement of key features such as stent strut patterns, coil configurations, or catheter tip designs. The ability to swiftly test and modify prototypes in a realistic environment dramatically reduces development time and costs, bringing innovative solutions to market more rapidly.
Enhanced Safety Profiling
Safety is paramount in medical device development, and aneurysm models play a crucial role in comprehensive safety profiling. These models allow for thorough assessment of potential failure modes and complications associated with new devices. Researchers can evaluate risks such as vessel perforation, device fracture, or unintended flow disruption in a controlled setting. This rigorous safety testing helps in identifying and mitigating potential risks before human trials, ultimately leading to safer devices and improved patient outcomes.
Bridging the Gap to Clinical Trials
Aneurysm models serve as a critical bridge between benchtop testing and clinical trials. By closely simulating in vivo conditions, these models provide valuable data that can inform the design of clinical trials and help in predicting real-world performance. This intermediate step allows researchers to refine protocols, optimize device selection criteria, and anticipate potential challenges in clinical application. The insights gained from extensive testing on aneurysm models can lead to more focused and efficient clinical trials, potentially reducing the time and resources required for bringing new devices to market.
Conclusion
Aneurysm models have emerged as indispensable tools in the realm of neurovascular device testing and innovation. Their ability to provide anatomically accurate, customizable, and physiologically relevant testing environments has revolutionized the development process for endovascular devices. From enhancing the precision of stent and catheter designs to accelerating the pace of innovation, these models are driving significant advancements in the treatment of cerebral aneurysms. As technology continues to evolve, the role of aneurysm models in shaping the future of neurovascular interventions remains paramount, promising improved outcomes for patients worldwide.
Contact Us
For cutting-edge aneurysm models that can elevate your research and development efforts, look no further than Trandomed. Our advanced 3D-printed medical simulators offer unparalleled realism and customization options to meet your specific needs. To explore how our models can benefit your neurovascular device testing and innovation projects, contact us at jackson.chen@trandomed.com.
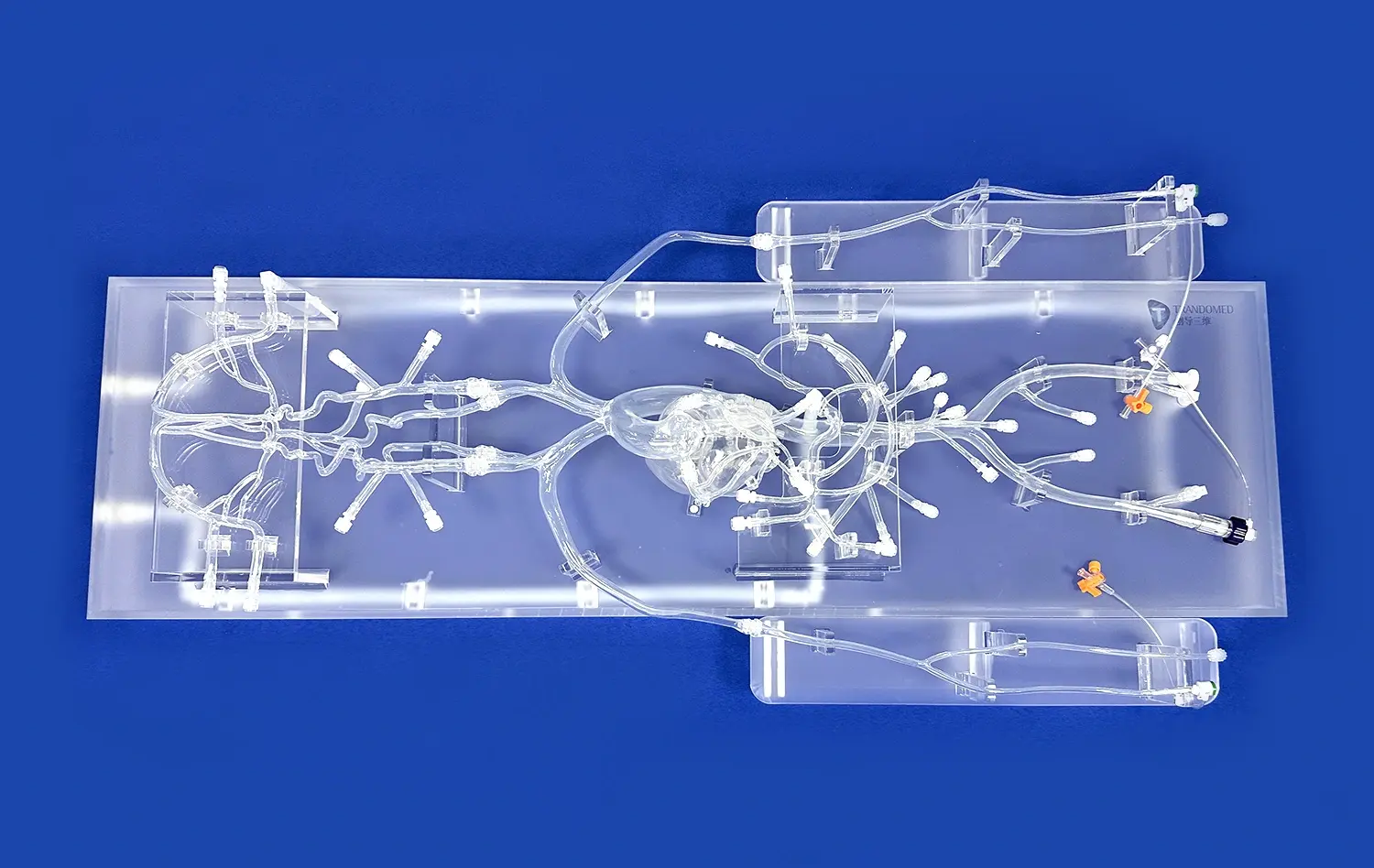
_1736214519364.webp)
 (SJ001D)_1734504338727.webp)