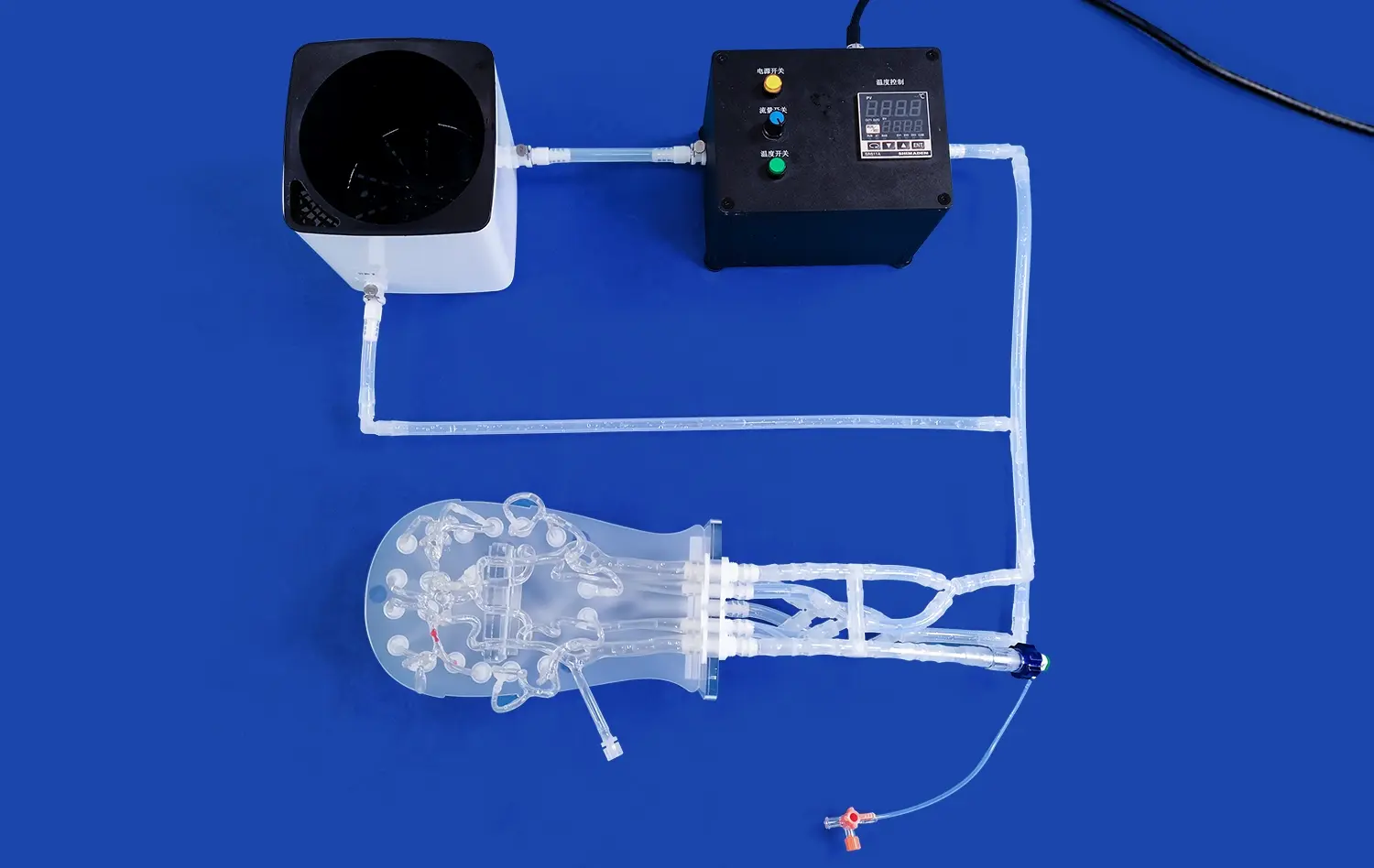
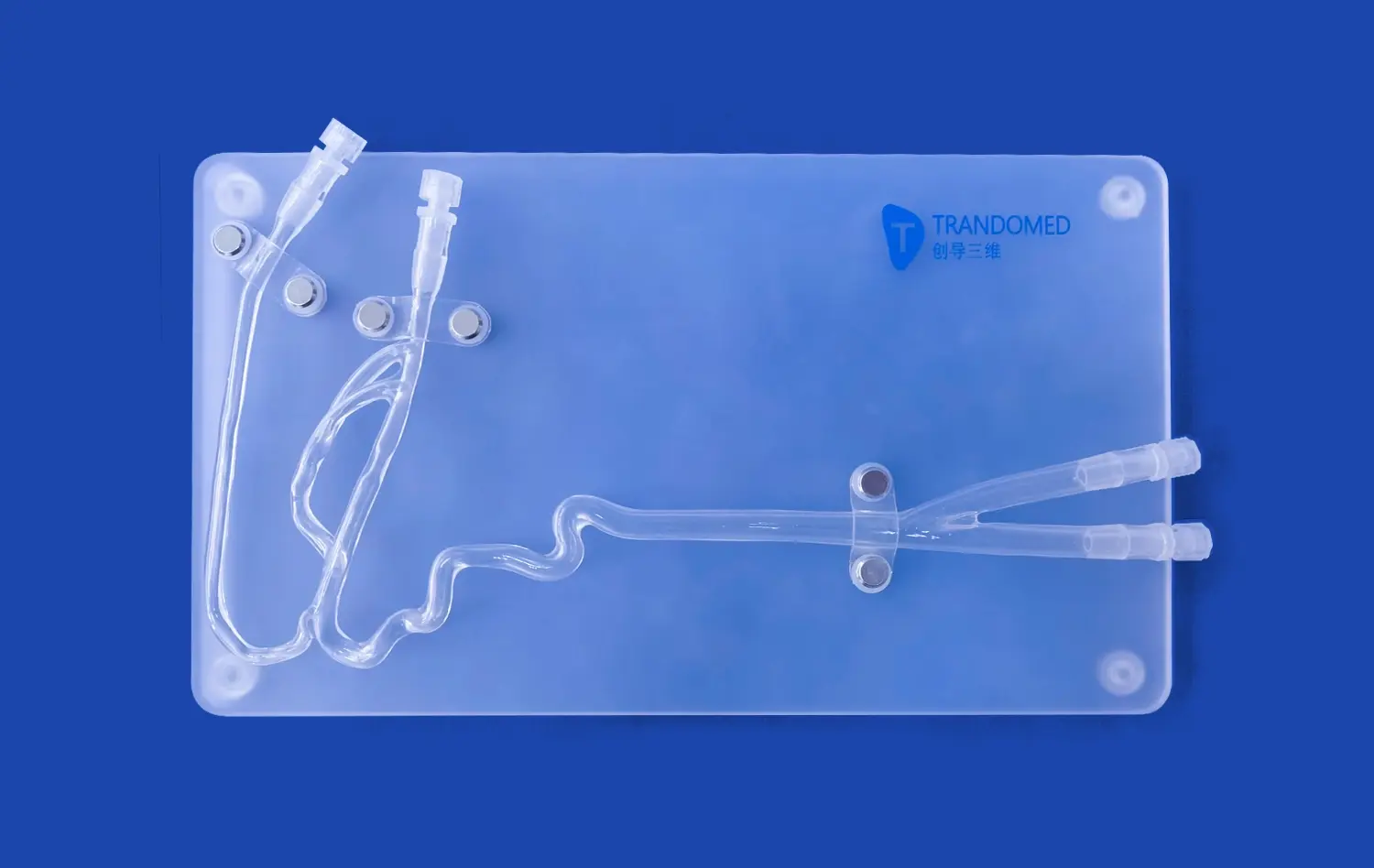
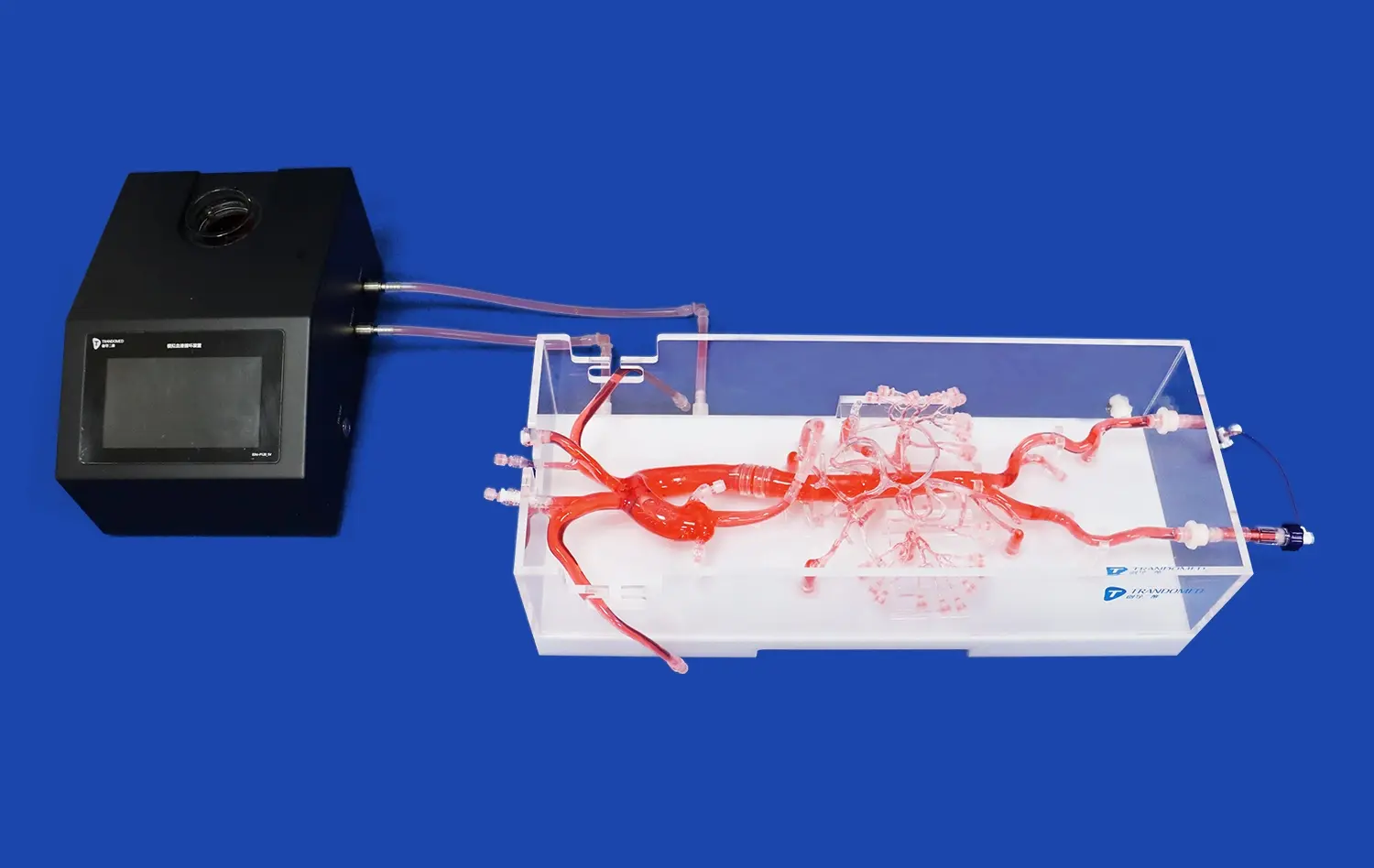
What Types of Abdominal Artery Devices Can Be Tested?
Stents and Grafts
The Aortic Arch Replaceable Model is particularly well-suited for testing various types of stents and grafts designed for abdominal artery applications. These devices, crucial in treating conditions such as abdominal aortic aneurysms (AAAs), can be thoroughly evaluated for their deployment accuracy, conformability to vessel walls, and overall performance within the simulated environment. The model's realistic anatomical features allow researchers to assess how these devices navigate through complex vascular structures, ensuring optimal placement and functionality.
Catheters and Guidewires
Interventional catheters and guidewires play a vital role in many abdominal artery procedures. The aortic arch replaceable model provides an excellent platform for testing the maneuverability, trackability, and pushability of these devices. Manufacturers can evaluate how their catheters and guidewires navigate through the intricate pathways of the aortic arch and abdominal arteries, helping to refine designs for improved performance in clinical settings.
Embolic Protection Devices
Embolic protection devices, designed to capture and remove debris during interventional procedures, can also be effectively tested using the Aortic Arch Replaceable Model. The model's ability to simulate realistic blood flow allows researchers to assess the efficacy of these devices in capturing embolic material and their impact on overall blood flow dynamics. This testing is crucial for ensuring the safety and effectiveness of procedures such as carotid artery stenting.
Benefits of Using a Replaceable Aortic Arch Model in Device Trials
Enhanced Accuracy and Reproducibility
One of the primary advantages of using a replaceable aortic arch model in device trials is the significantly enhanced accuracy and reproducibility of results. The model's precise anatomical details, based on real human CT and MRI data, provide a consistent and reliable testing environment. This consistency allows researchers to conduct multiple trials under identical conditions, ensuring that any variations in device performance are due to the device itself rather than inconsistencies in the testing platform. The replaceable components also enable researchers to maintain the model's integrity over multiple tests, further enhancing the reliability of results.
Cost-Effective and Efficient Testing
The aortic arch replaceable model offers a cost-effective and efficient solution for device testing. By providing a reusable platform that can be easily reconfigured for different testing scenarios, it significantly reduces the need for multiple, single-use models or animal studies. This not only lowers the overall cost of testing but also accelerates the development process. Researchers can quickly iterate through design modifications and performance evaluations without the logistical challenges and ethical considerations associated with in vivo testing. The model's durability and replaceable components ensure a long service life, further enhancing its cost-effectiveness.
Versatility in Simulating Pathological Conditions
The versatility of the Aortic Arch Replaceable Model in simulating various pathological conditions is a significant benefit for device trials. Trandomed offers customization options that allow researchers to replicate specific vascular abnormalities, such as aneurysms, stenoses, or embolisms, in different locations within the model. This capability enables device manufacturers to test their products under a wide range of clinically relevant scenarios, ensuring that devices are effective across diverse patient populations and pathological presentations. The ability to simulate these conditions in a controlled environment provides valuable insights into device performance and potential limitations before progressing to clinical trials.
Simulating Realistic Blood Flow for Accurate Device Evaluation
Advanced Flow Dynamics Replication
The Aortic Arch Replaceable Model excels in replicating advanced flow dynamics, crucial for accurate device evaluation. By incorporating precise vessel geometries and material properties that mimic human tissue, the model creates a realistic environment for studying blood flow patterns. This capability is essential for assessing how devices interact with blood flow, particularly in regions of complex geometry such as the aortic arch. Researchers can observe phenomena like flow separation, recirculation zones, and wall shear stress, which are critical factors in device performance and potential complications such as thrombosis or restenosis.
Integration with Imaging Modalities
A key feature of the aortic arch replaceable model is its compatibility with various imaging modalities commonly used in clinical practice. The model's design allows for direct use in quantitative blood flow analysis using techniques such as CT angiography (CTA), digital subtraction angiography (DSA), magnetic resonance angiography (MRA), optical coherence tomography (OCT), particle image velocimetry (PIV), and Doppler ultrasound. This integration enables researchers to evaluate devices under conditions that closely mimic real-world clinical imaging scenarios, providing valuable insights into how devices will appear and perform during actual procedures. The ability to use these imaging techniques also allows for detailed analysis of flow patterns and device-tissue interactions, further enhancing the accuracy of device evaluations.
Customizable Flow Parameters
The Aortic Arch Replaceable Model offers the ability to customize flow parameters, allowing researchers to simulate a wide range of physiological and pathological conditions. By adjusting factors such as flow rate, pressure, and pulsatility, researchers can recreate specific hemodynamic scenarios relevant to their device testing needs. This flexibility is particularly valuable when evaluating devices designed for specific patient populations or clinical conditions. For example, researchers can simulate the altered flow dynamics associated with aortic stenosis or the increased flow rates typical of exercise conditions. The ability to fine-tune these parameters ensures that devices are tested under the most relevant and challenging conditions they may encounter in clinical use.
Conclusion
The aortic arch replaceable model for Abdominal Artery Device Testing represents a significant advancement in medical device evaluation. Its ability to simulate realistic anatomical structures and blood flow dynamics provides an unparalleled platform for testing a wide range of abdominal artery devices. The model's versatility, cost-effectiveness, and compatibility with various imaging modalities make it an invaluable tool for device manufacturers, researchers, and clinicians. By enabling more accurate and comprehensive testing, this innovative model contributes to the development of safer and more effective interventional devices, ultimately leading to improved patient outcomes in vascular medicine.
Contact Us
For more information about the Aortic Arch Replaceable Model and how it can benefit your device testing or research needs, please contact Trandomed. Our team of experts is ready to provide you with customized solutions that meet your specific requirements. Experience the future of medical device testing with Trandomed's advanced 3D printed silicone medical simulators. Contact us at jackson.chen@trandomed.com to learn more about our innovative products and services.
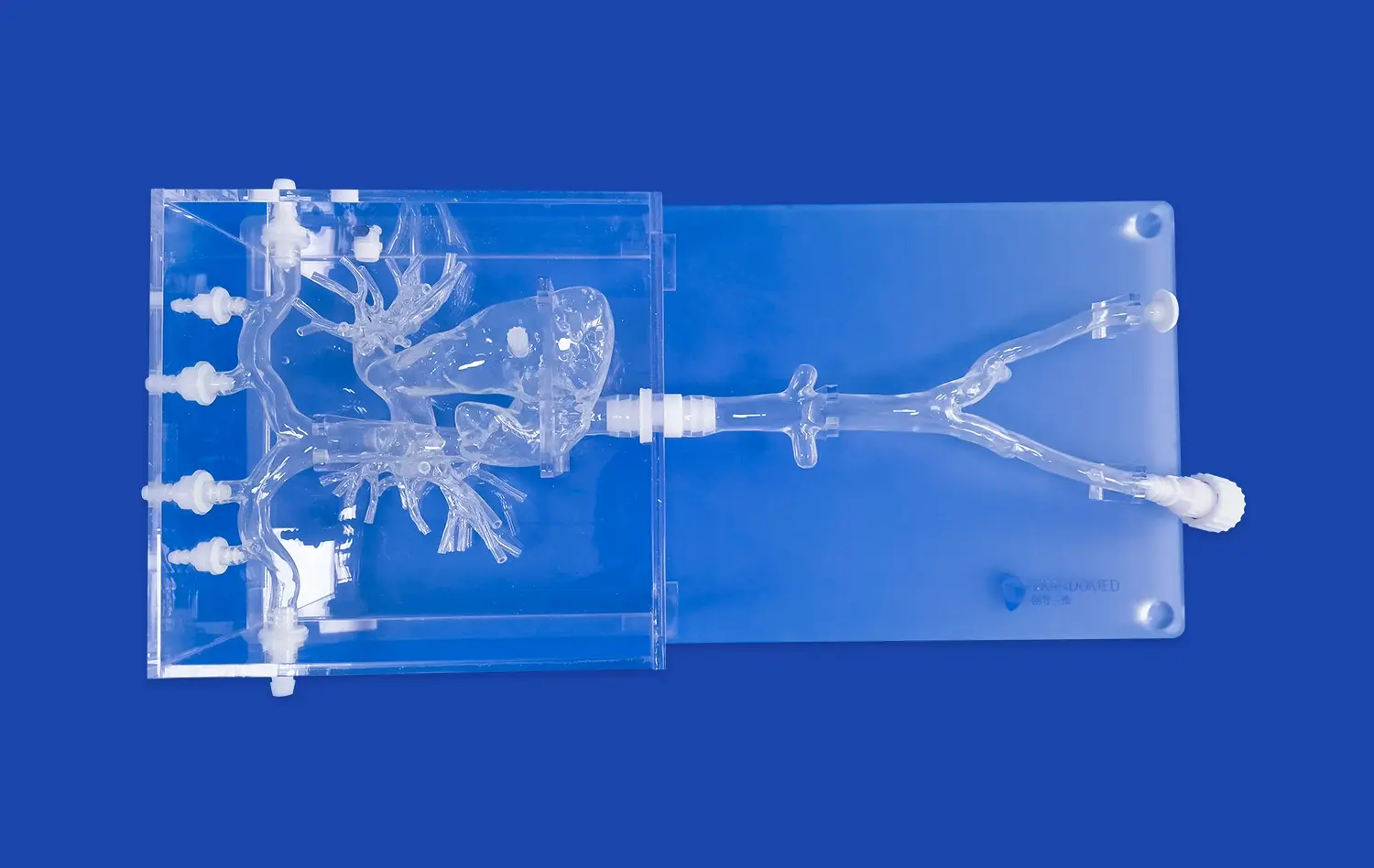
_1734507815464.webp)
_1732863713705.webp)