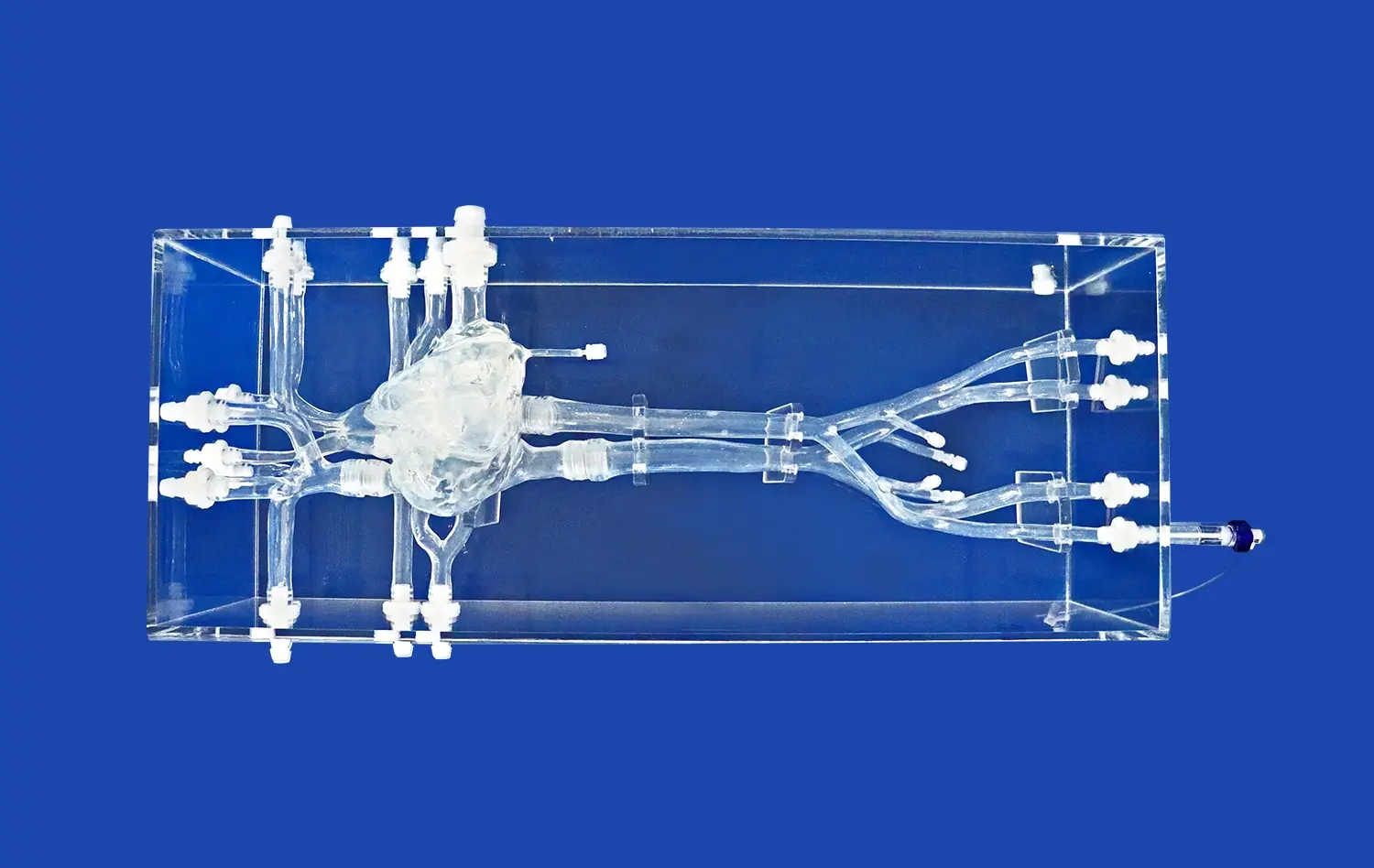
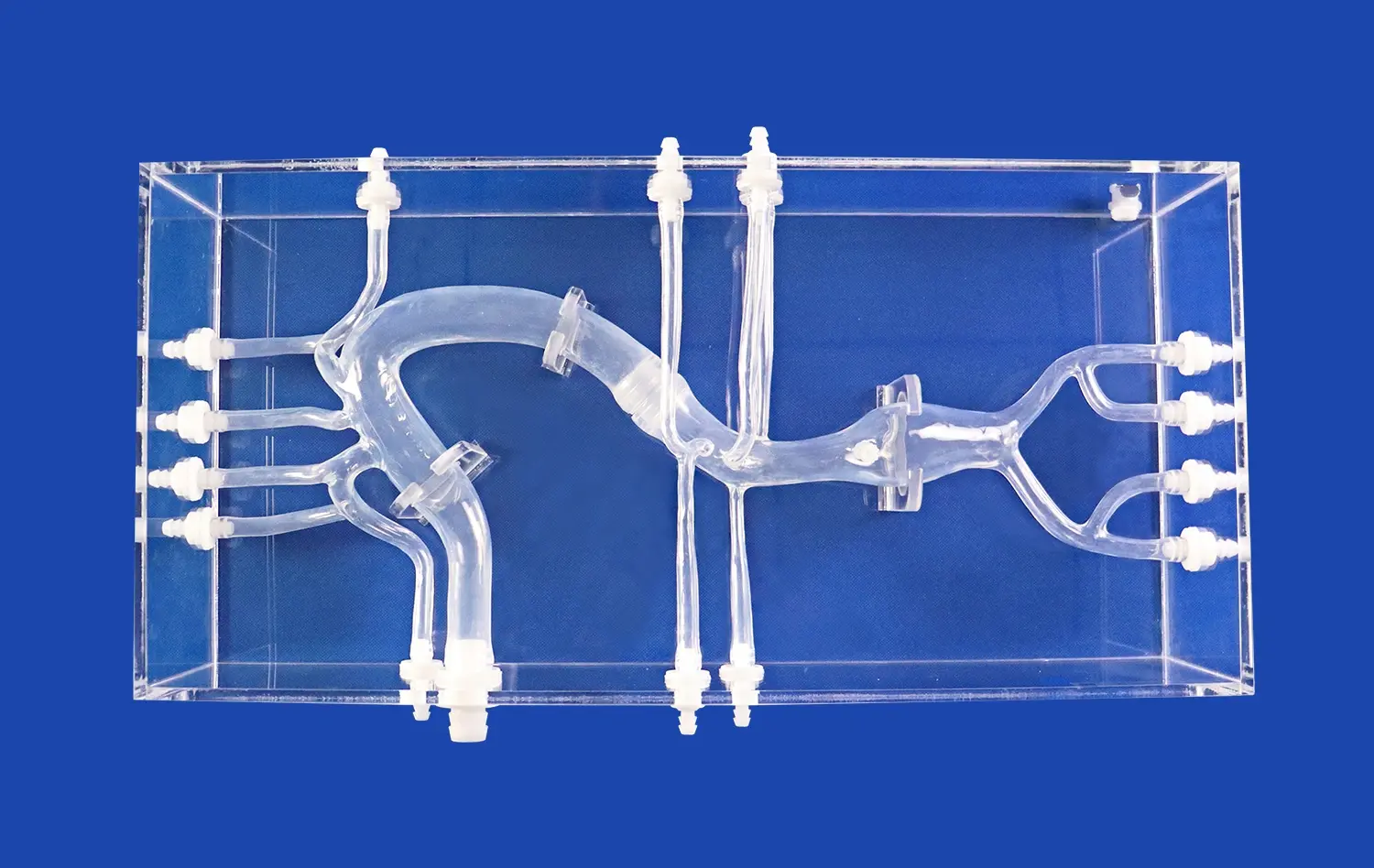
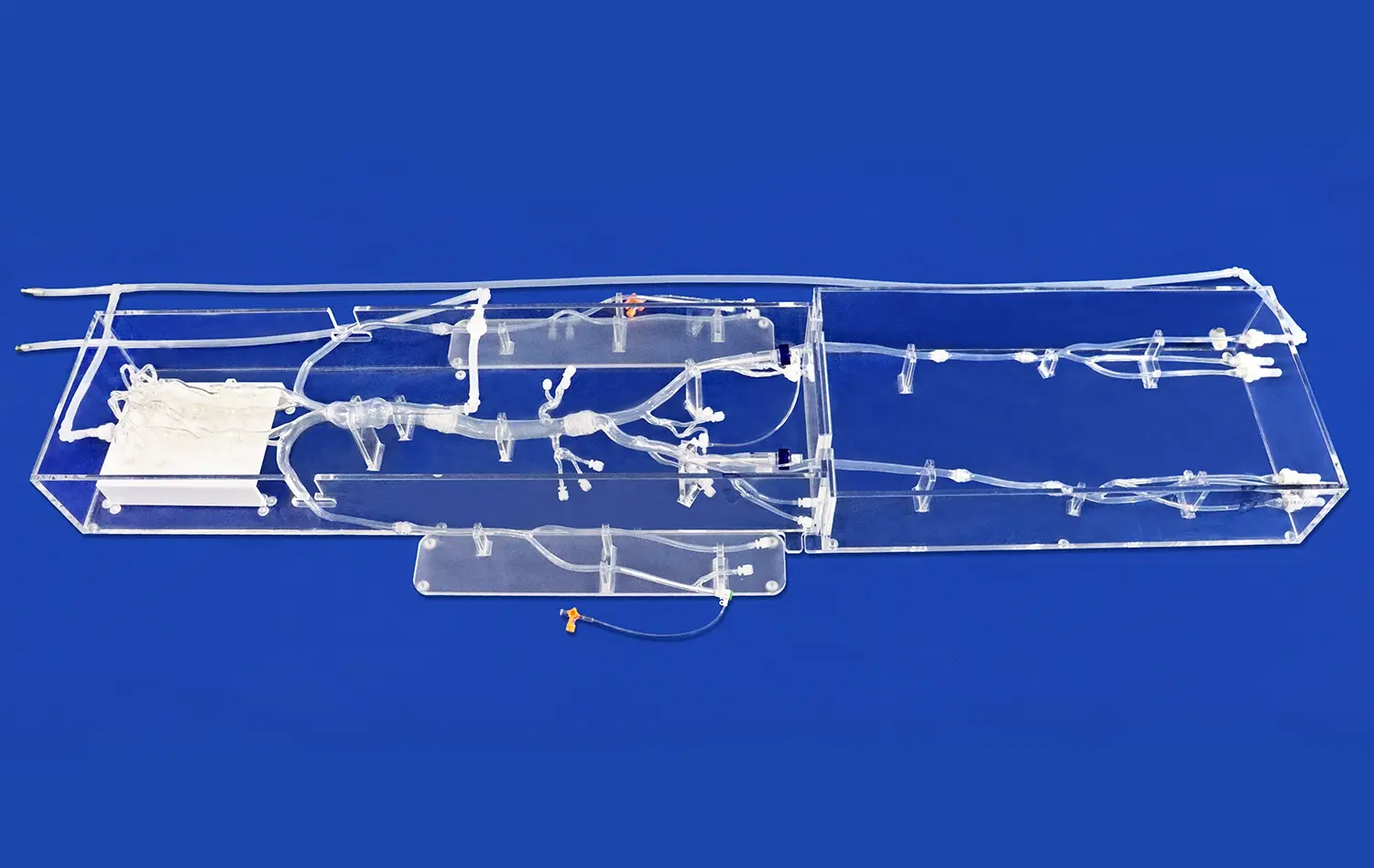
How Can the Model Showcase Device Capabilities Effectively?
Realistic Anatomical Representation
The Aortic Arch Replaceable Model excels in showcasing device capabilities through its highly accurate anatomical representation. Built on real human CT and MRI data using reverse 3D reconstruction technology, the model provides an exceptionally realistic environment for device testing and demonstration. This level of anatomical precision allows medical professionals and device manufacturers to evaluate how their interventional devices interact with complex vascular structures, closely mimicking real-world conditions.
Versatile Testing Scenarios
One of the key strengths of the model lies in its ability to accommodate a wide range of testing scenarios. The modular design, featuring detachable components such as the left ventricle, thoracic aorta, and abdominal aorta, allows for the simulation of various clinical situations. This versatility enables comprehensive testing of different interventional devices, from guidewires and catheters to stents and transcatheter aortic valve delivery systems. By replicating diverse anatomical variations and pathological conditions, the model provides a platform for thorough evaluation of device performance across multiple scenarios.
Enhanced Visualization
The model's design incorporates features that enhance visualization during device demonstrations. Transparent components and strategically placed viewing windows allow observers to clearly see device navigation and deployment within the simulated vascular structures. This improved visibility is crucial for showcasing the precision and effectiveness of interventional devices, particularly in challenging anatomical regions like the aortic arch. The ability to observe device-tissue interactions in real-time provides valuable insights into device behavior and performance.
Features That Facilitate Realistic Demonstrations
Customizable Pathologies
A standout feature of the Aortic Arch Replaceable Model is its ability to incorporate customizable pathologies. This flexibility allows for the simulation of various clinical conditions, such as aortic aneurysms, stenoses, and embolisms. By replicating these pathological states, the model enables device manufacturers to demonstrate how their products perform in specific disease scenarios. This capability is particularly valuable for showcasing specialized interventional devices designed to address particular vascular abnormalities.
Material Properties
The model's construction utilizes advanced materials that closely mimic the properties of human tissue. The silicone composition, with a Shore 40A durometer, provides a realistic feel and response during device insertion and manipulation. This attention to material properties ensures that the tactile feedback experienced during demonstrations closely resembles that of actual interventional procedures. The lifelike tissue response enhances the value of the model for both training purposes and device capability demonstrations.
Integrated Fluid Dynamics
To further enhance realism, the Aortic Arch Replaceable Model can be integrated with fluid dynamics systems. This feature allows for the simulation of blood flow within the model, providing a more comprehensive environment for device testing. The ability to replicate hemodynamic conditions is crucial for evaluating how interventional devices perform under various flow states. This capability is particularly valuable for demonstrating the effectiveness of devices designed to address flow-related vascular issues.
Improving Stakeholder Understanding Through Hands-On Simulation
Interactive Learning Experience
The Aortic Arch Replaceable Model offers an unparalleled interactive learning experience for stakeholders. By providing hands-on access to a realistic anatomical model, it allows medical professionals, device manufacturers, and regulatory bodies to gain a deeper understanding of interventional procedures and device capabilities. This tactile engagement helps bridge the gap between theoretical knowledge and practical application, fostering a more comprehensive grasp of the complexities involved in interventional cardiology.
Comparative Analysis
One of the model's strengths lies in its ability to facilitate comparative analysis of different interventional devices. Stakeholders can directly observe and compare the performance of various devices within the same anatomical context. This side-by-side evaluation provides valuable insights into the relative strengths and potential limitations of different interventional technologies. Such comparative demonstrations are instrumental in informing decision-making processes for healthcare providers, researchers, and regulatory agencies.
Risk Assessment and Mitigation
The Aortic Arch Replaceable Model serves as an excellent tool for risk assessment and mitigation in interventional device development. By allowing stakeholders to simulate challenging scenarios and potential complications, the model helps identify and address potential risks associated with device use. This proactive approach to risk management not only enhances device safety but also builds confidence among stakeholders regarding the reliability and effectiveness of interventional technologies.
Conclusion
The Aortic Arch Replaceable Model represents a significant advancement in interventional device demonstration and evaluation. Its realistic anatomical representation, customizable features, and versatile applications make it an indispensable tool for medical education, device development, and stakeholder engagement. By providing a platform for hands-on simulation and comprehensive testing, the model enhances understanding, facilitates innovation, and ultimately contributes to improved patient outcomes in interventional cardiology.
Contact Us
Experience the unparalleled realism and versatility of Trandomed's Aortic Arch Replaceable Model. Our cutting-edge 3D printed silicone medical simulators offer precise anatomical accuracy, customizable pathologies, and seamless integration with your specific training or demonstration needs. Elevate your interventional device showcases and stakeholder engagement with our advanced models. For more information or to discuss your custom requirements, contact us at jackson.chen@trandomed.com.
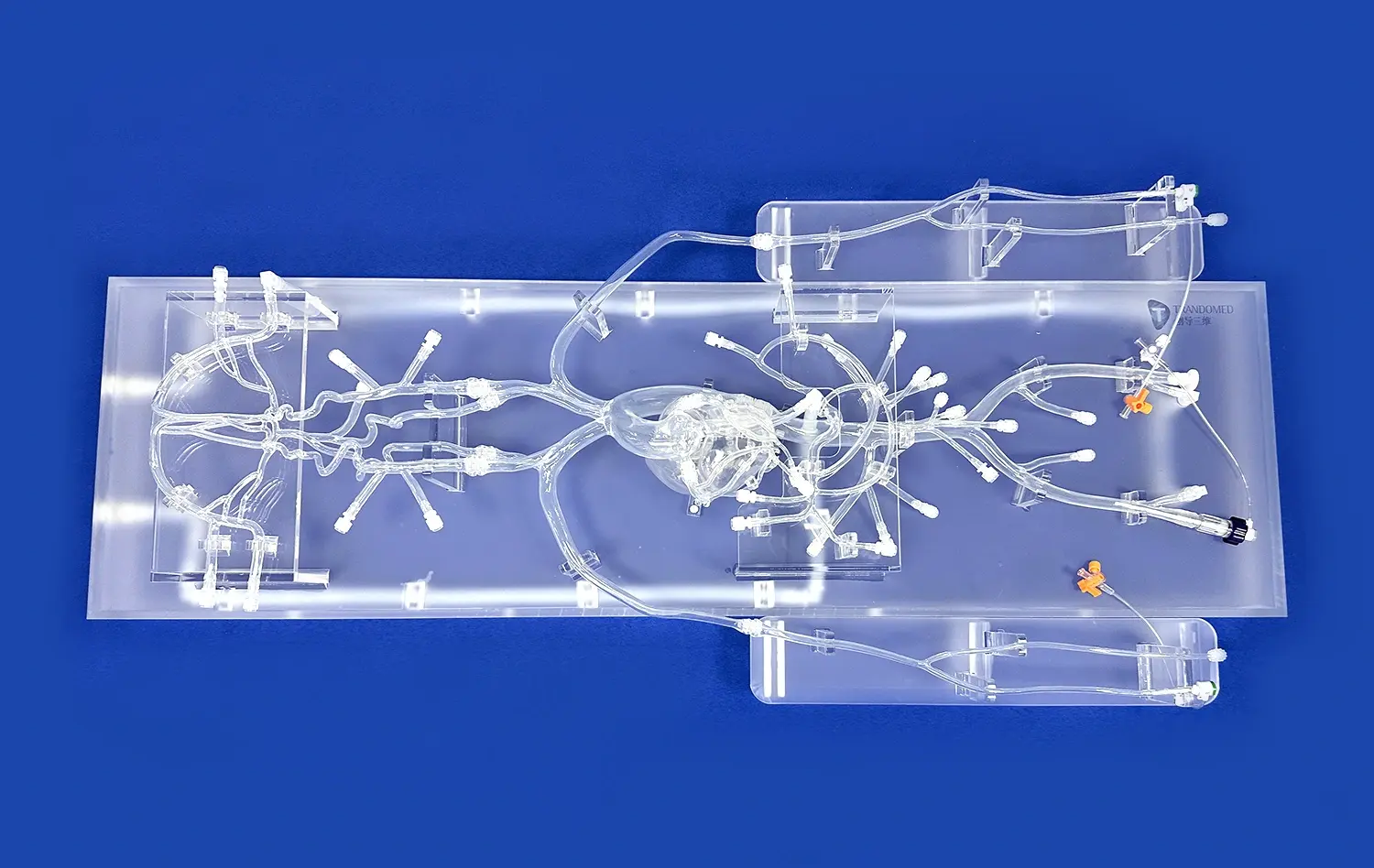

 (SJ001D)_1734504338727.webp)