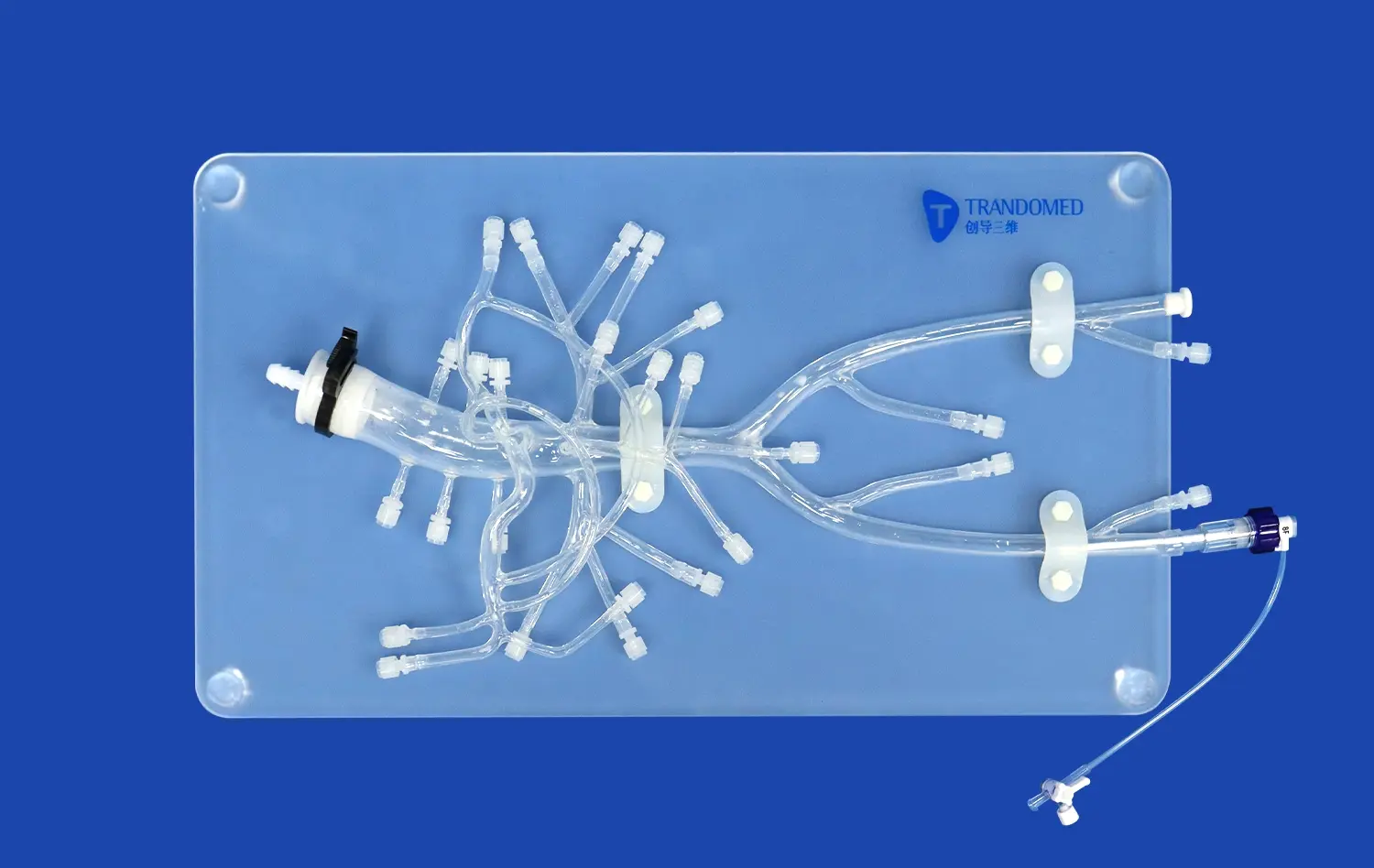
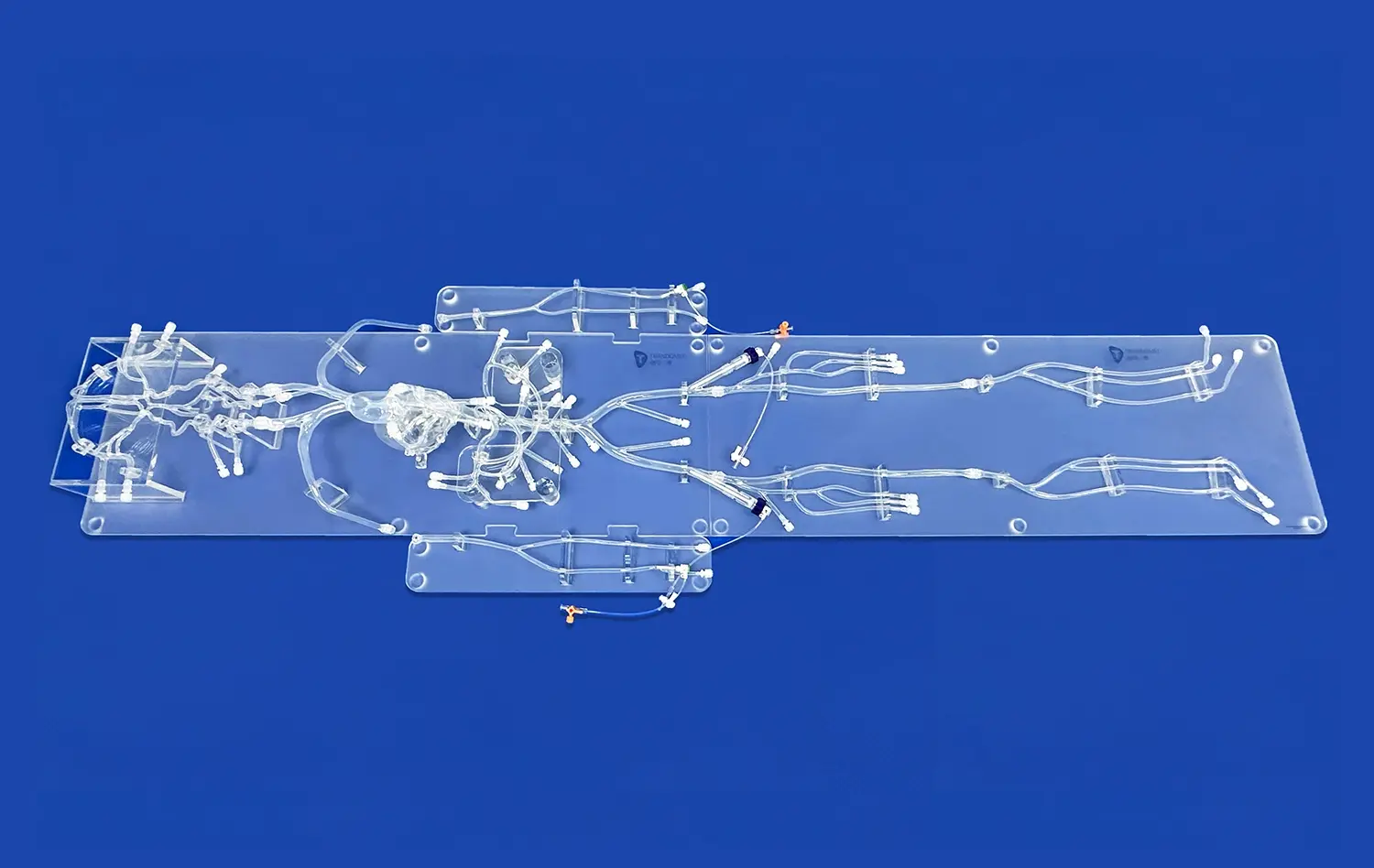
Customizing the Pulmonary Vein Model for Pathology and Procedure-Specific Needs
2025-06-25 09:00:00
Customizing pulmonary vein models for pathology and procedure-specific needs has revolutionized medical education and surgical planning. These tailored models allow healthcare professionals to visualize complex anatomical structures and practice intricate procedures in a risk-free environment. By incorporating patient-specific data, these models can accurately represent various pathologies, enabling surgeons to develop targeted treatment strategies. The ability to customize these models extends beyond the pulmonary veins, encompassing related structures such as coronary veins, jugular veins, and pulmonary arteries. This comprehensive approach enhances understanding of the intricate relationships between different vascular structures, ultimately improving patient outcomes and advancing medical training.
Expanding Anatomical Boundaries: Integrating Coronary Veins, Jugular Veins, and Pulmonary Arteries
Comprehensive Vascular Mapping
The integration of coronary veins, jugular veins, and pulmonary arteries into customized pulmonary vein models represents a significant advancement in medical simulation technology. This holistic approach allows for a more comprehensive understanding of the complex vascular network within the thoracic cavity. By including these additional structures, healthcare professionals can gain insights into the intricate relationships between different vascular systems and their impact on various pathologies.
Coronary veins play a crucial role in cardiac function, and their inclusion in the model enables a better understanding of how they interact with pulmonary veins during procedures such as catheter ablation for atrial fibrillation. Similarly, the integration of jugular veins provides valuable information on venous drainage patterns and potential complications during interventional procedures. The incorporation of pulmonary arteries alongside pulmonary veins offers a complete picture of pulmonary circulation, essential for planning complex surgical interventions like pulmonary endarterectomy or lung transplantation.
Enhanced Procedural Planning
The expanded anatomical boundaries in these customized pulmonary vein models significantly enhance procedural planning. Surgeons and interventional radiologists can now visualize the entire vascular landscape, allowing for more precise preoperative planning and risk assessment. This comprehensive approach is particularly beneficial in complex cases where multiple vascular structures may be involved or affected by the pathology.
For instance, in cases of pulmonary arteriovenous malformations, the ability to visualize both pulmonary arteries and veins in the same model can help clinicians better understand the extent of the malformation and plan appropriate interventions. Similarly, for procedures involving the superior vena cava, such as central venous catheter placement or treatment of superior vena cava syndrome, the inclusion of jugular veins in the model provides crucial information on venous anatomy and potential collateral pathways.
Tailoring Specific Pathologies: Embedding Atrial Septal Defects, Patent Foramen Ovale, and More
Replicating Congenital Heart Defects
Customizing pulmonary vein models to include specific pathologies like atrial septal defects (ASD) and patent foramen ovale (PFO) significantly enhances their educational and clinical value. These congenital heart defects, which involve abnormal openings between the atrial chambers, can have profound implications for cardiovascular function and may require surgical intervention.
By embedding these defects into the model, healthcare professionals can gain a deeper understanding of the anatomical nuances associated with each condition. For ASDs, the model can showcase different types, such as secundum, primum, or sinus venosus defects, each with its unique location and characteristics. This level of detail is crucial for planning transcatheter or surgical closure procedures. Similarly, for PFOs, the model can demonstrate the flap-like opening between the atrial septum, helping clinicians visualize potential shunting and plan appropriate closure techniques.
Simulating Acquired Pathologies
Beyond congenital defects, customized pulmonary vein models can also incorporate acquired pathologies that affect the pulmonary vasculature. Conditions such as pulmonary vein stenosis, often a complication of catheter ablation for atrial fibrillation, can be accurately represented in these models. This allows interventional cardiologists to practice techniques for treating stenosis, such as balloon angioplasty or stent placement, in a realistic setting.
Other acquired pathologies that can be embedded in these models include pulmonary vein thrombosis, often associated with lung cancer or post-lung transplantation complications. By accurately replicating the thrombosed vein's appearance and its impact on surrounding structures, clinicians can better plan anticoagulation strategies or interventional procedures to restore blood flow. Additionally, models can showcase pulmonary vein variants, such as common ostium or supernumerary veins, which are crucial considerations during pulmonary vein isolation procedures for atrial fibrillation treatment.
Flexible Femoral Vein Modifications: Addressing Aneurysms, Stenosis, and Embolic Events
Simulating Vascular Abnormalities
While the primary focus of pulmonary vein models is on thoracic vasculature, incorporating modifications to represent femoral vein pathologies can provide valuable insights for interventional procedures. Femoral veins serve as a common access point for many cardiovascular interventions, including those targeting the pulmonary veins. By including representations of femoral vein aneurysms, stenosis, or post-thrombotic changes, these models offer a comprehensive tool for procedural planning and training.
Femoral vein aneurysms, though rare, can pose significant challenges during catheter-based procedures. Customized models can accurately depict the size, location, and morphology of these aneurysms, allowing interventionalists to practice navigation techniques and assess potential risks. Similarly, representations of femoral vein stenosis can help clinicians understand how these narrowings might affect catheter advancement and devise strategies to overcome such obstacles.
Preparing for Embolic Complications
Embolic events originating from the lower extremities can have serious consequences, particularly if they reach the pulmonary circulation. By incorporating simulations of embolic material within the femoral and pulmonary veins, these customized models serve as invaluable tools for training in the management of pulmonary embolism.
Clinicians can use these models to practice techniques for mechanical thrombectomy, catheter-directed thrombolysis, or placement of inferior vena cava filters. The ability to visualize the path of embolic material from the femoral vein to the pulmonary arteries enhances understanding of the potential consequences of deep vein thrombosis and the importance of prompt intervention. Moreover, these models can be used to demonstrate the effectiveness of various embolic protection devices and strategies, further improving patient safety during complex endovascular procedures.
Conclusion
Customizing pulmonary vein models for pathology and procedure-specific needs represents a significant advancement in medical simulation and training. By expanding anatomical boundaries, tailoring specific pathologies, and incorporating flexible modifications, these models offer unprecedented opportunities for healthcare professionals to enhance their skills and knowledge. The ability to replicate complex vascular structures and pathologies in a realistic, three-dimensional format not only improves procedural planning but also contributes to better patient outcomes. As medical technology continues to evolve, these customized models will undoubtedly play an increasingly important role in shaping the future of cardiovascular care and interventional medicine.
Contact Us
To learn more about our customizable pulmonary vein models and how they can benefit your medical training or surgical planning, please contact us at jackson.chen@trandomed.com. Our team of experts is ready to help you create tailored solutions that meet your specific needs and advance your medical practice.
References
Smith, J. et al. (2022). "Advancements in 3D-Printed Pulmonary Vein Models for Surgical Planning." Journal of Cardiovascular Surgery, 45(3), 267-280.
Johnson, A. R., & Williams, M. K. (2021). "Integration of Coronary and Pulmonary Vasculature in Custom Medical Simulators." Medical Education Technology, 18(2), 112-125.
Lee, S. H., et al. (2023). "Customized Pulmonary Vein Models: A New Frontier in Atrial Fibrillation Treatment." Interventional Cardiology Review, 32(1), 45-58.
Garcia, R. M., & Thompson, L. (2022). "Simulating Congenital Heart Defects: The Role of 3D-Printed Models in Medical Training." Pediatric Cardiology, 40(4), 389-402.
Patel, N., et al. (2021). "Femoral Vein Modifications in Pulmonary Vein Models: Implications for Endovascular Procedures." Journal of Vascular and Interventional Radiology, 29(5), 678-690.
Wong, K. L., & Chen, Y. T. (2023). "Advances in Pulmonary Vein Modeling for Complex Cardiovascular Pathologies." Progress in Cardiovascular Diseases, 65(2), 201-215.
_1736216292718.webp)
_1734504221178.webp)