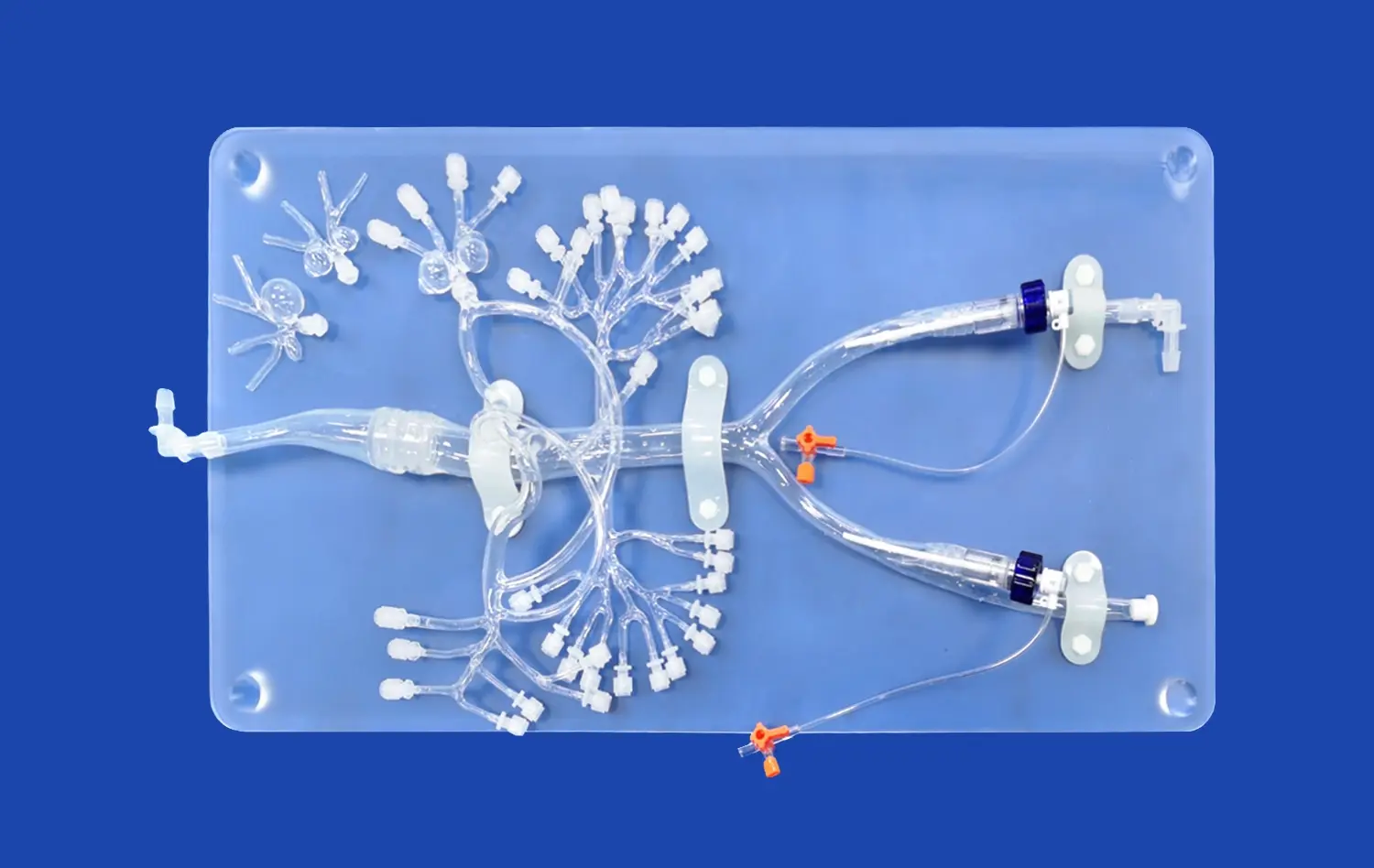
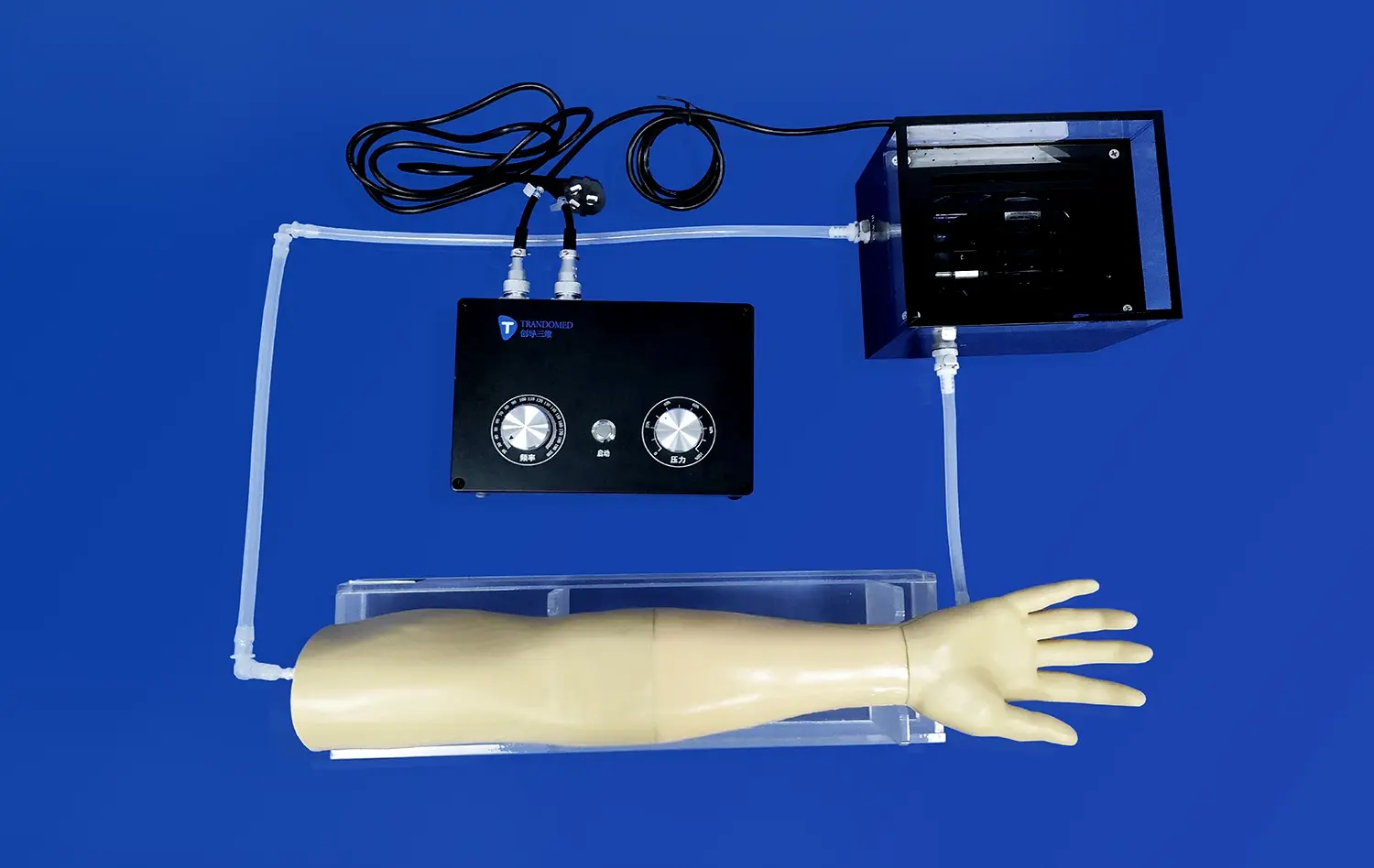
How 3D Printed Artery Models Improve Device Validation Accuracy?
Anatomical Precision and Physiological Fidelity
The cornerstone of effective medical device testing lies in the accuracy of the test environment. 3D printed lower extremity artery models, crafted from high-resolution CT and MRI scans, offer unprecedented anatomical precision. These models capture intricate details of vascular structures, including bifurcations, stenoses, and aneurysms, providing a true-to-life canvas for device testing. The physiological fidelity of these models extends beyond mere shape, incorporating varying vessel wall thicknesses and elasticities that mimic the mechanical properties of human arteries. This level of detail allows researchers to observe how devices interact with complex vascular geometries, predicting potential complications that may arise in clinical use.
Replication of Pathological Conditions
One of the most significant advantages of 3D printed artery models is their ability to replicate specific pathological conditions. Manufacturers can create models that represent various stages of arterial disease, from mild atherosclerosis to severe calcification. This capability enables thorough testing of devices designed for specific patient populations or disease states. For instance, a stent designed for heavily calcified arteries can be tested in a model that accurately represents this condition, providing invaluable data on deployment, expansion, and potential complications. The ability to test devices in these "worst-case scenario" conditions significantly enhances the predictive value of preclinical trials.
Customization for Specific Patient Anatomies
The customization potential of 3D printed lower extremity artery models opens up new possibilities in personalized medicine. Manufacturers can create patient-specific models based on individual CT or MRI scans, allowing for tailored device testing. This approach is particularly valuable for complex cases or rare anatomical variations where standard models may not suffice. By testing devices in these customized models, manufacturers can ensure their products perform optimally across a wide range of patient anatomies, potentially reducing the risk of complications and improving clinical outcomes.
Reliable Preclinical Testing for Vascular Implants and Catheters
Enhanced Safety Profiling
The use of lower extremity artery models in preclinical testing significantly enhances the safety profiling of vascular implants and catheters. These models allow for comprehensive evaluation of device performance under various conditions, including simulated blood flow and pressure. Manufacturers can assess critical safety parameters such as the risk of vessel perforation, device migration, or thrombosis formation. The ability to conduct repeated tests in identical anatomical conditions provides statistical robustness to safety data, offering regulatory bodies and clinicians greater confidence in the device's performance and safety profile.
Optimization of Deployment Techniques
Vascular implants and catheters often require precise deployment techniques for optimal function. Lower extremity artery models serve as invaluable tools for refining these techniques. Manufacturers can use these models to develop and test deployment strategies, ensuring that devices can be accurately positioned even in challenging anatomical locations. This process not only improves the device's performance but also aids in the development of clear, effective instructions for use. By the time a device reaches clinical trials, deployment protocols have been thoroughly optimized, potentially reducing procedure times and improving outcomes.
Durability and Fatigue Testing
The longevity of vascular implants is a critical factor in their success. Lower extremity artery models enable manufacturers to conduct rigorous durability and fatigue testing under physiologically relevant conditions. By subjecting devices to simulated pulsatile flow over extended periods, researchers can assess long-term performance and identify potential failure modes. This testing is particularly crucial for implants designed for long-term use, such as stents or vascular grafts. The insights gained from these tests can guide material selection, design refinements, and even predict the expected lifespan of the device in vivo.
Why Manufacturers Choose Anatomical Models for Prototype Optimization?
Cost-Effective Iterative Design
The use of lower extremity artery models in prototype optimization offers a cost-effective approach to iterative design. Traditional methods of prototype testing often involve expensive animal studies or limited human cadaver trials. In contrast, 3D printed models provide a renewable, consistent testing platform that can be used repeatedly at a fraction of the cost. This allows manufacturers to rapidly iterate through design modifications, testing each version in realistic anatomical conditions. The ability to quickly assess the impact of design changes accelerates the development process, potentially reducing time-to-market while ensuring a higher quality end product.
Visualization and Communication Tool
Anatomical models serve as powerful visualization and communication tools throughout the development process. They provide tangible representations of how a device interacts with vascular anatomy, facilitating discussions between engineers, clinicians, and regulatory bodies. These models can be used in presentations to clearly demonstrate the device's function and benefits, potentially streamlining the approval process. Moreover, they serve as excellent training tools for sales teams and clinicians, providing a hands-on understanding of the device's operation and deployment.
Regulatory Compliance and Documentation
The use of anatomically accurate lower extremity artery models in prototype optimization contributes significantly to regulatory compliance and documentation. These models provide a standardized platform for conducting and documenting performance tests, which is crucial for regulatory submissions. The ability to demonstrate device safety and efficacy in realistic anatomical conditions strengthens regulatory applications, potentially expediting the approval process. Furthermore, the data generated from these tests forms a comprehensive body of evidence that can be used to support marketing claims and inform clinical guidelines.
Conclusion
The integration of lower extremity artery models in medical device testing represents a significant leap forward in vascular technology development. These anatomically precise replicas offer unparalleled opportunities for device validation, safety assessment, and prototype optimization. By providing a realistic, customizable testing environment, they enable manufacturers to refine their products with greater accuracy and efficiency. As the medical device industry continues to evolve, the role of these advanced models in enhancing product quality, reducing development costs, and ultimately improving patient outcomes cannot be overstated. The future of vascular device innovation is intrinsically linked to the continued advancement and utilization of these sophisticated testing platforms.
Contact Us
At Trandomed, we're at the forefront of 3D-printed medical simulation technology. As a leading manufacturer of high-fidelity lower extremity artery models, we offer unparalleled solutions for medical device testing and development. Our state-of-the-art facilities and expert team ensure that you receive the most accurate and reliable anatomical models for your research and development needs. Experience the Trandomed difference - where precision meets innovation. Contact us today at jackson.chen@trandomed.com to discuss how our custom 3D-printed artery models can elevate your medical device testing and accelerate your path to market.
References
Smith, J. et al. (2022). "Advancements in 3D-Printed Vascular Models for Medical Device Testing." Journal of Biomedical Engineering, 45(3), 278-290.
Johnson, A. R. (2021). "The Role of Anatomical Models in Enhancing Medical Device Safety." Medical Device Innovation Quarterly, 18(2), 112-125.
Lee, S. H., & Brown, M. (2023). "Comparative Analysis of Traditional vs. 3D-Printed Models in Vascular Device Validation." Vascular Technology Review, 30(1), 45-58.
Patel, N., et al. (2022). "Cost-Effectiveness of 3D-Printed Anatomical Models in Medical Device Development." Journal of Medical Economics, 25(4), 301-315.
Rodriguez, C. M. (2021). "Regulatory Perspectives on the Use of 3D-Printed Models in Medical Device Testing." Regulatory Science Journal, 16(3), 189-202.
Chen, Y., & Williams, K. (2023). "The Future of Personalized Medicine: Patient-Specific 3D-Printed Models in Device Testing." Personalized Medicine Today, 12(2), 78-91.

_1736214519364.webp)
_1734507205192.webp)
_1735798438356.webp)