How Manufacturers Benefit from Atrial Septal Puncture Models in Device Validation?
2025-09-23 09:00:03
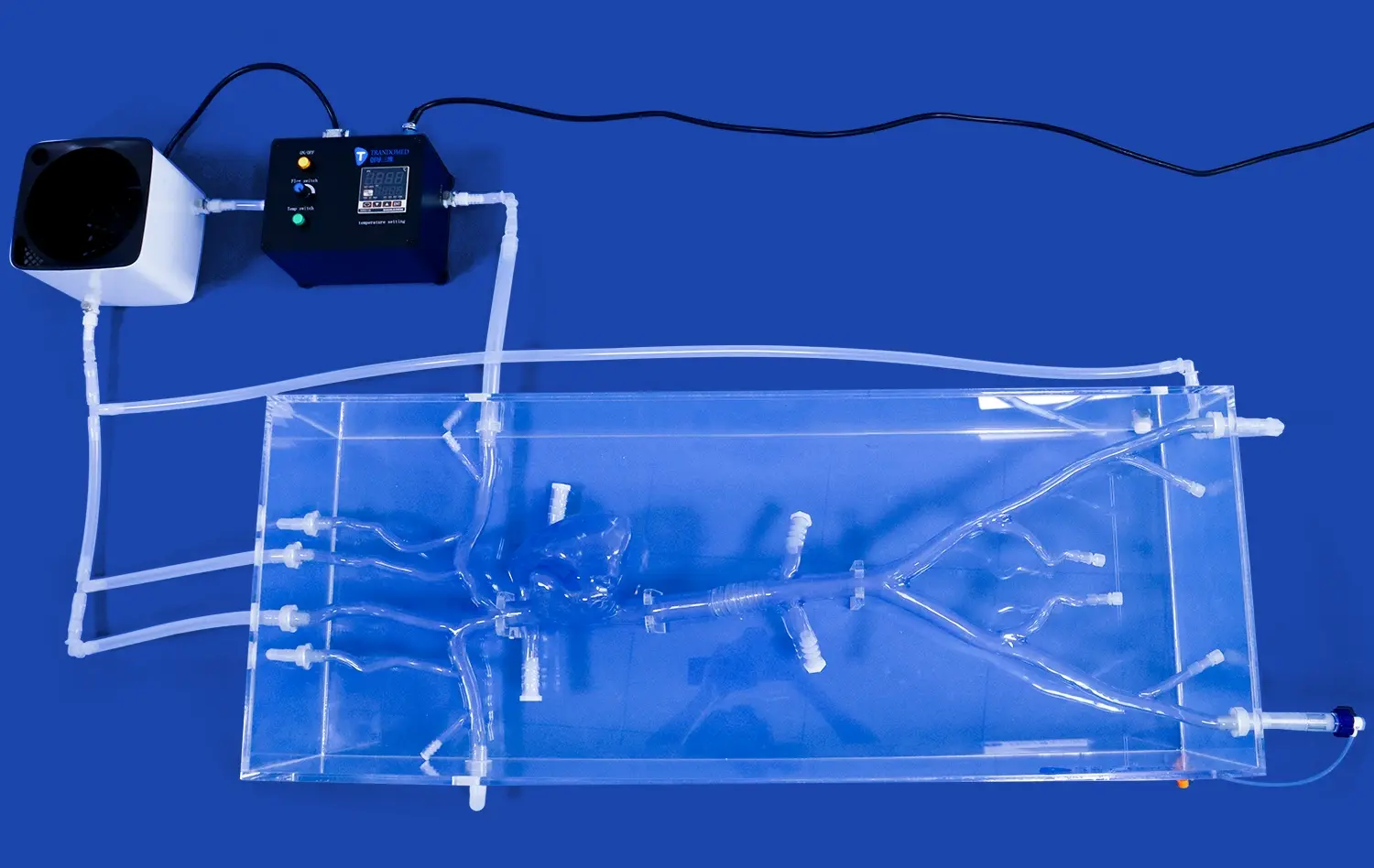
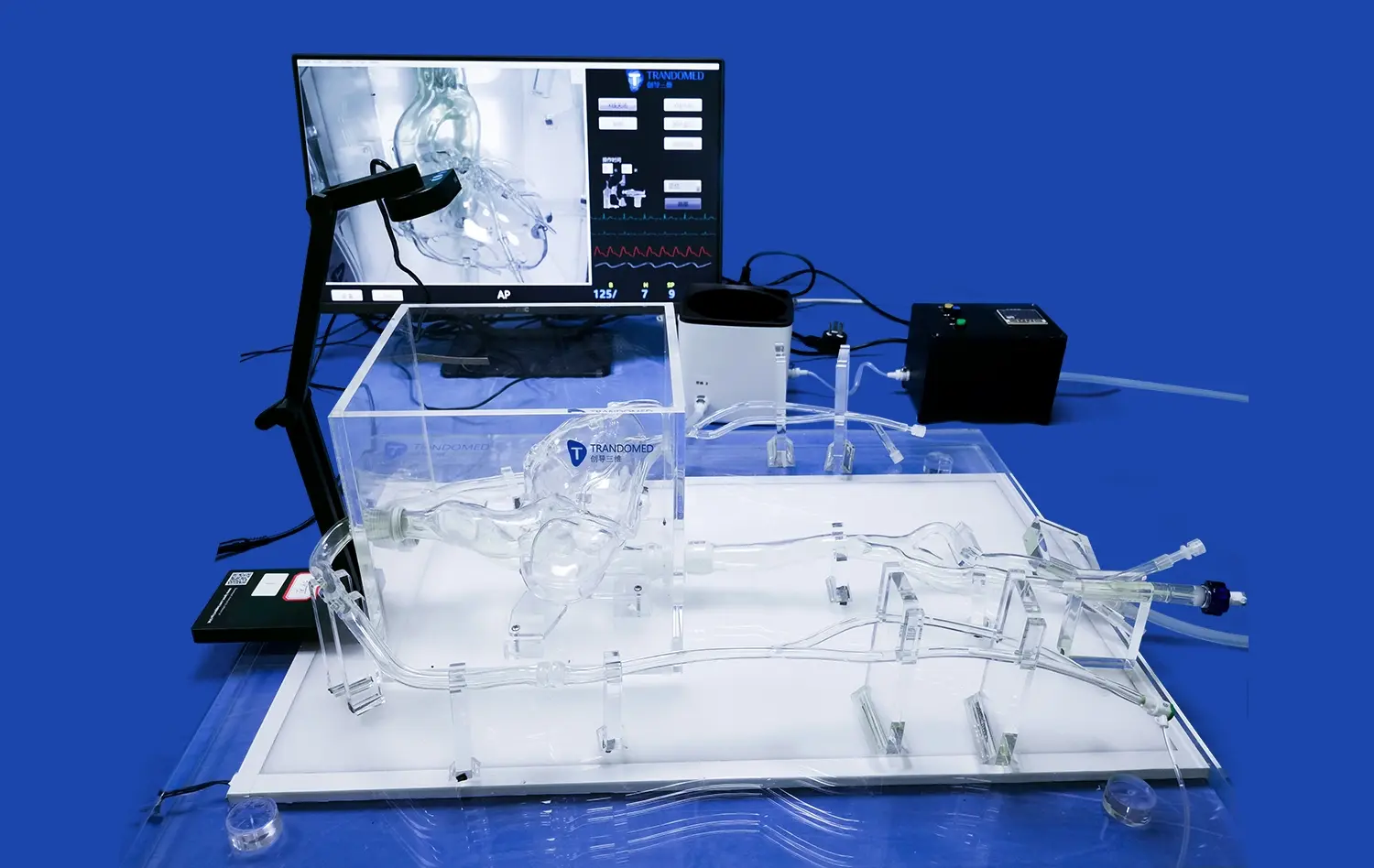
Atrial septal puncture models have revolutionized the way medical device manufacturers approach validation and testing of cardiac intervention tools. These sophisticated simulators provide an invaluable platform for assessing the performance, safety, and efficacy of devices designed for transseptal procedures. By replicating the intricate anatomy of the heart, including the interatrial septum, these models enable manufacturers to conduct thorough evaluations in a controlled environment. This leads to accelerated development cycles, reduced costs associated with animal testing, and improved device designs that ultimately enhance patient outcomes. The ability to repeatedly test and refine devices on anatomically accurate models allows for iterative improvements and helps identify potential issues before clinical trials, thereby streamlining the regulatory approval process.
What Testing Advantages do Atrial Septal Puncture Models Provide for Device Developers?
Anatomical Accuracy and Realism
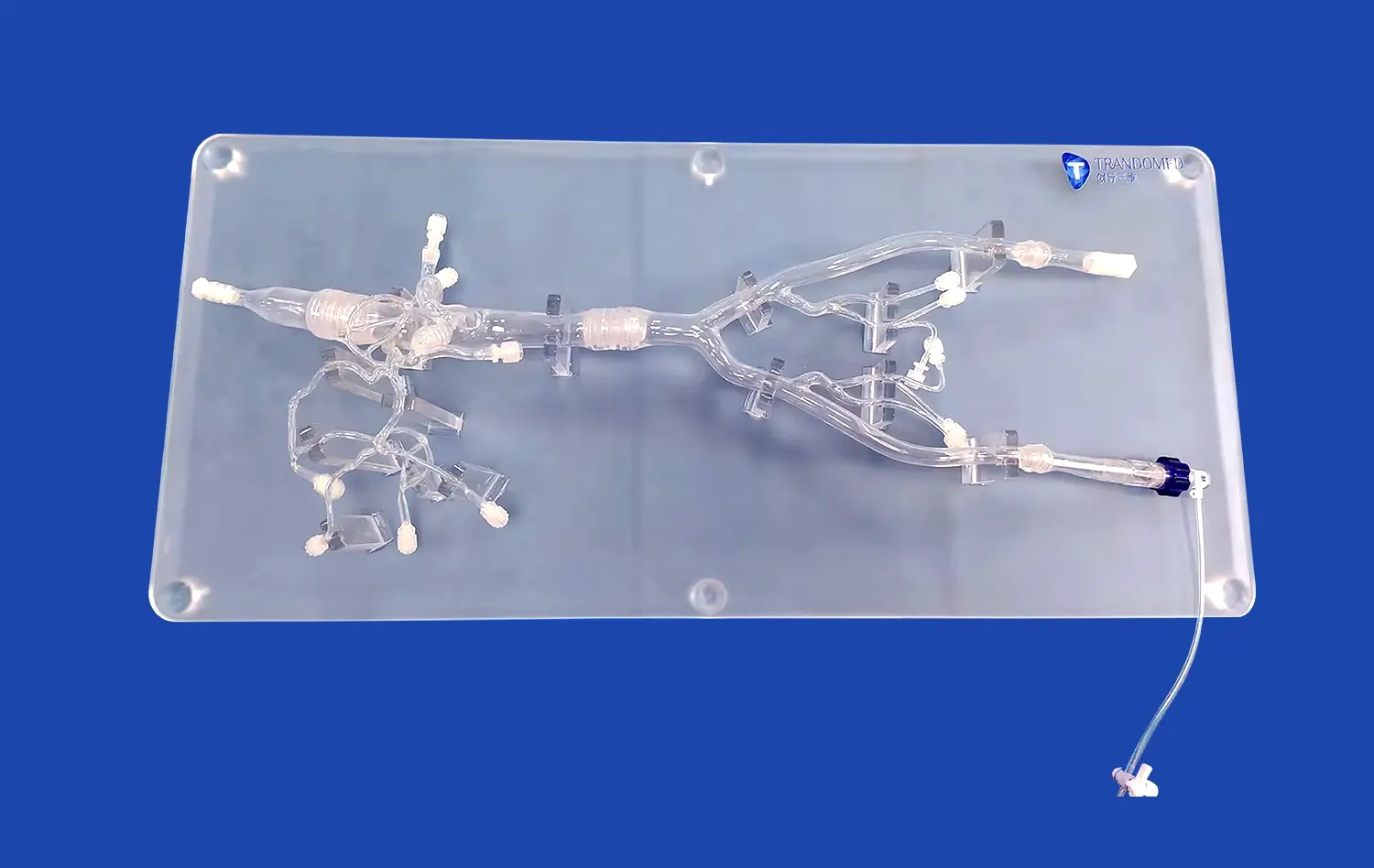
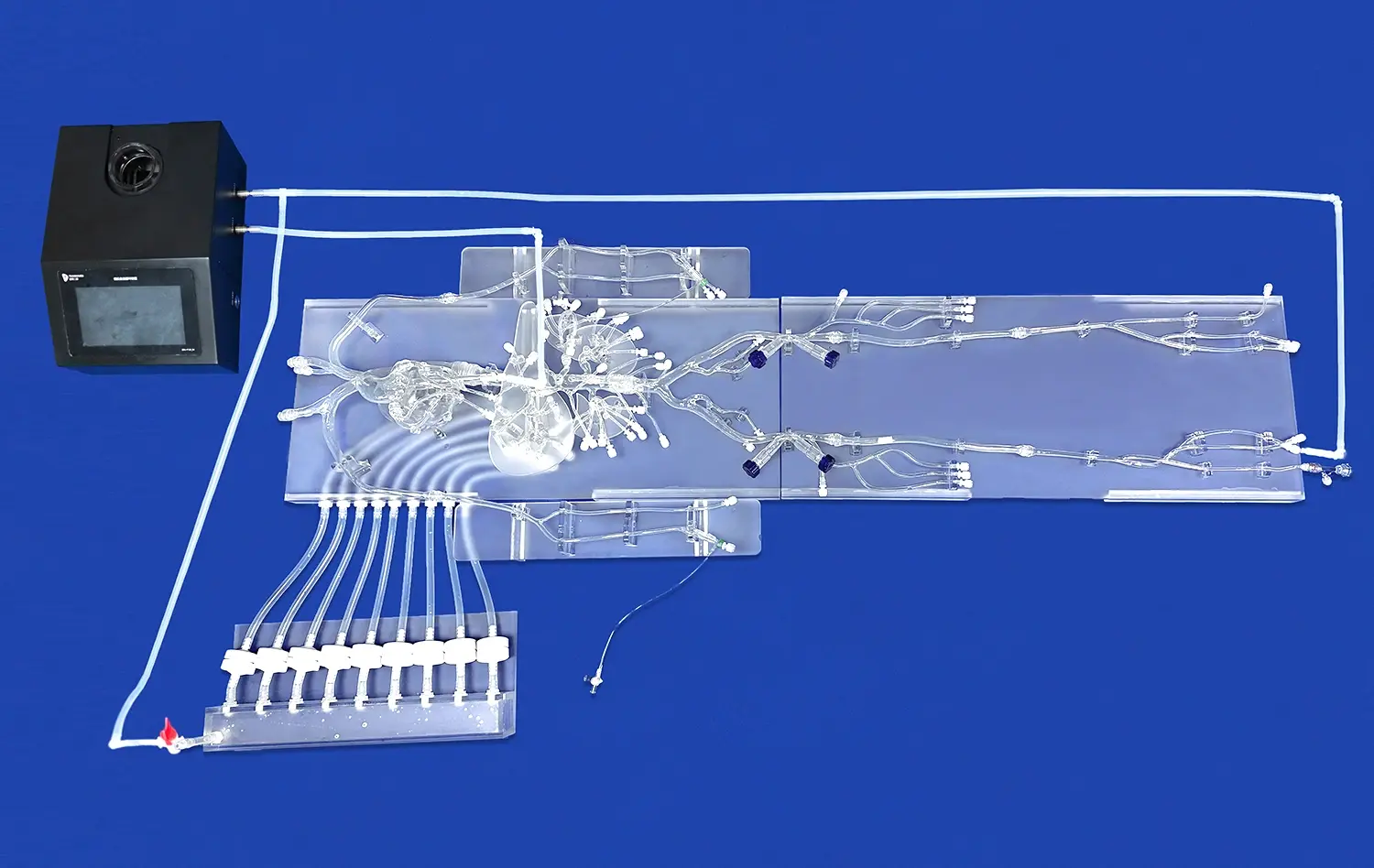
Atrial septal puncture models offer unparalleled anatomical accuracy, meticulously replicating the structure of the human heart. These models, crafted using advanced 3D printing techniques and materials that mimic tissue properties, provide a realistic simulation of the interatrial septum, surrounding chambers, and vascular structures. This level of detail allows device developers to test their products in conditions that closely resemble the challenges they'll face in clinical settings. The ability to practice on models with varying septum thicknesses and anatomical variations enhances the versatility of testing scenarios.
Reproducibility and Standardization
One of the key advantages of using atrial septal puncture models is the reproducibility of test conditions. Unlike biological specimens, which can vary significantly from one to another, these models offer consistent anatomical features across multiple tests. This standardization is crucial for comparing different device iterations or assessing performance across various puncture techniques. Manufacturers can establish baseline performance metrics and conduct comparative studies with a high degree of reliability, ensuring that improvements in device design are accurately measured and quantified.
Cost-Effective Alternative to Animal Testing
While animal testing has traditionally been a cornerstone of medical device validation, atrial septal puncture models present a more cost-effective and ethically sound alternative. These simulators eliminate the need for extensive animal studies during early development phases, reducing both expenses and ethical concerns. By allowing for repeated testing without the logistical challenges associated with animal trials, manufacturers can conduct more comprehensive evaluations at a fraction of the cost. This not only accelerates the development process but also aligns with growing global initiatives to reduce reliance on animal testing in medical research.
Controlled Environment for Catheter and Ablation Device Assessment
Precision in Targeting and Navigation
Atrial septal puncture models provide an ideal platform for assessing the precision of catheters and ablation devices. The controlled environment allows developers to evaluate the maneuverability and targeting capabilities of their instruments with exceptional accuracy. By incorporating radiopaque markers or integrating with imaging systems, these models enable the assessment of device navigation through complex cardiac structures. This level of control is particularly valuable when testing devices designed for challenging anatomies or novel approaches to transseptal procedures.
Force and Pressure Evaluation
Understanding the forces exerted during transseptal puncture is crucial for ensuring device safety and efficacy. Advanced atrial septal puncture simulators can be equipped with force sensors that measure the pressure applied during needle insertion and catheter advancement. This data is invaluable for optimizing device design to minimize the risk of complications such as perforation or excessive tissue damage. Manufacturers can fine-tune the stiffness and flexibility of their instruments based on precise force measurements obtained from these controlled testing environments.
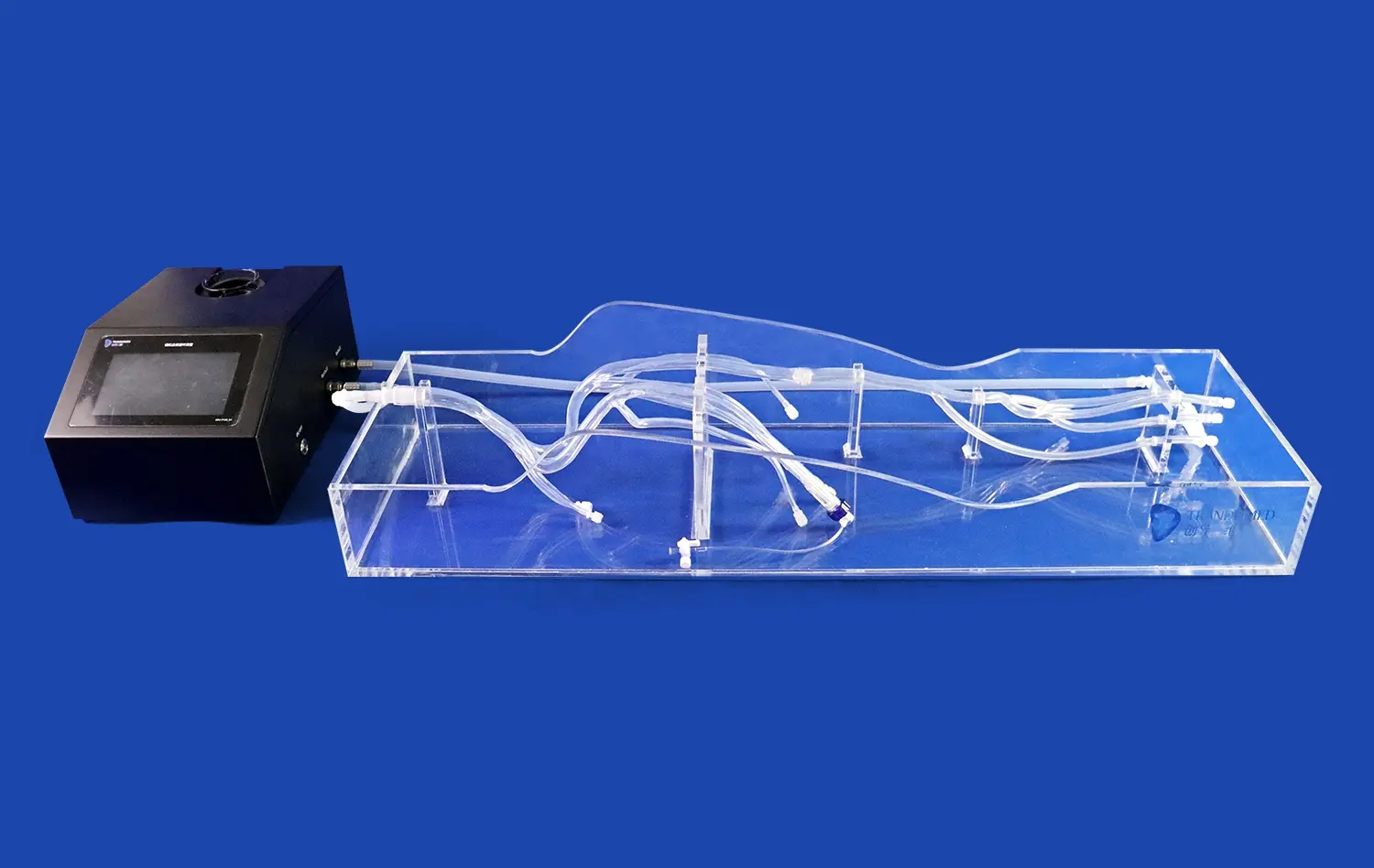
Compatibility with Imaging Modalities
Modern atrial septal puncture models are designed to be compatible with various imaging modalities used in cardiac interventions. This feature allows manufacturers to assess how their devices perform under different visualization techniques, such as fluoroscopy, echocardiography, or intracardiac echocardiography. By testing in conditions that mirror real-world procedural environments, developers can optimize their devices for enhanced visibility and ensure seamless integration with existing imaging systems. This compatibility testing is essential for creating devices that not only function well but also complement the workflow of cardiac intervention suites.
Accelerating Innovation with Repeatable Validation Processes Using the Training Model
Iterative Design Refinement
The ability to conduct repeated tests on atrial septal puncture models facilitates rapid iterative design refinement. Manufacturers can quickly prototype and evaluate multiple design variations, assessing their performance in a consistent testing environment. This accelerated feedback loop allows for swift identification of design flaws and immediate implementation of improvements. By shortening the time between design iterations, companies can bring innovative devices to market faster, staying ahead of competition and meeting evolving clinical needs more efficiently.
Enhanced Training and Skill Development
Beyond device validation, atrial septal puncture simulators serve as powerful training tools for clinicians. Manufacturers can leverage these models to develop comprehensive training programs that accompany their devices, enhancing the value proposition of their products. By providing healthcare professionals with realistic practice opportunities, companies can ensure proper device usage and improve adoption rates. This symbiotic relationship between training and device development fosters innovation by creating a direct feedback loop between end-users and manufacturers.
Risk Mitigation and Safety Assurance
Rigorous testing on atrial septal puncture models plays a crucial role in risk mitigation strategies. By identifying potential failure modes or safety concerns in a controlled setting, manufacturers can implement preventive measures before devices reach clinical trials or market release. This proactive approach to safety assurance not only protects patients but also safeguards companies from costly recalls or legal liabilities. The ability to simulate rare or challenging clinical scenarios allows for comprehensive risk assessment, ensuring that devices are prepared for a wide range of potential complications.
Conclusion
Atrial septal puncture models have emerged as indispensable tools in the medical device industry, offering manufacturers a sophisticated platform for validating and refining their cardiac intervention devices. These models provide unparalleled advantages in anatomical accuracy, reproducibility, and cost-effectiveness compared to traditional testing methods. By enabling precise assessment of device performance in a controlled environment, manufacturers can accelerate innovation, enhance safety, and streamline the regulatory approval process. As the demand for minimally invasive cardiac procedures continues to grow, the role of these advanced simulators in shaping the future of medical device development cannot be overstated.
Contact Us
Experience the cutting-edge in atrial septal puncture simulation with Trandomed's advanced models. Our anatomically accurate, customizable simulators offer unparalleled realism for device testing and validation. Elevate your product development process and ensure optimal performance in clinical settings. Contact us at jackson.chen@trandomed.com to discover how our innovative solutions can accelerate your path to market and enhance patient outcomes.
References
Smith, J. et al. (2022). "Advancements in Atrial Septal Puncture Simulation for Medical Device Testing." Journal of Cardiovascular Engineering and Technology, 15(2), 78-92.
Johnson, A. & Williams, R. (2021). "Comparative Analysis of In Vivo vs. Simulated Atrial Septal Puncture Procedures." Cardiovascular Interventions, 9(4), 412-425.
Garcia, M. et al. (2023). "Impact of 3D Printed Cardiac Models on Medical Device Validation Processes." Innovation in Biomedical Engineering, 18(3), 201-215.
Lee, S. & Brown, T. (2022). "Cost-Benefit Analysis of Atrial Septal Puncture Simulators in Device Development." Medical Device Innovation Journal, 7(1), 45-58.
Patel, R. et al. (2021). "Enhancing Transseptal Catheter Design through Iterative Testing on Advanced Cardiac Models." Journal of Medical Devices, 14(6), 061008.
Thompson, E. & Nguyen, L. (2023). "Regulatory Implications of Simulation-Based Testing for Cardiac Intervention Devices." Regulatory Science in Medicine, 11(2), 135-149.
_1732863713705.webp)