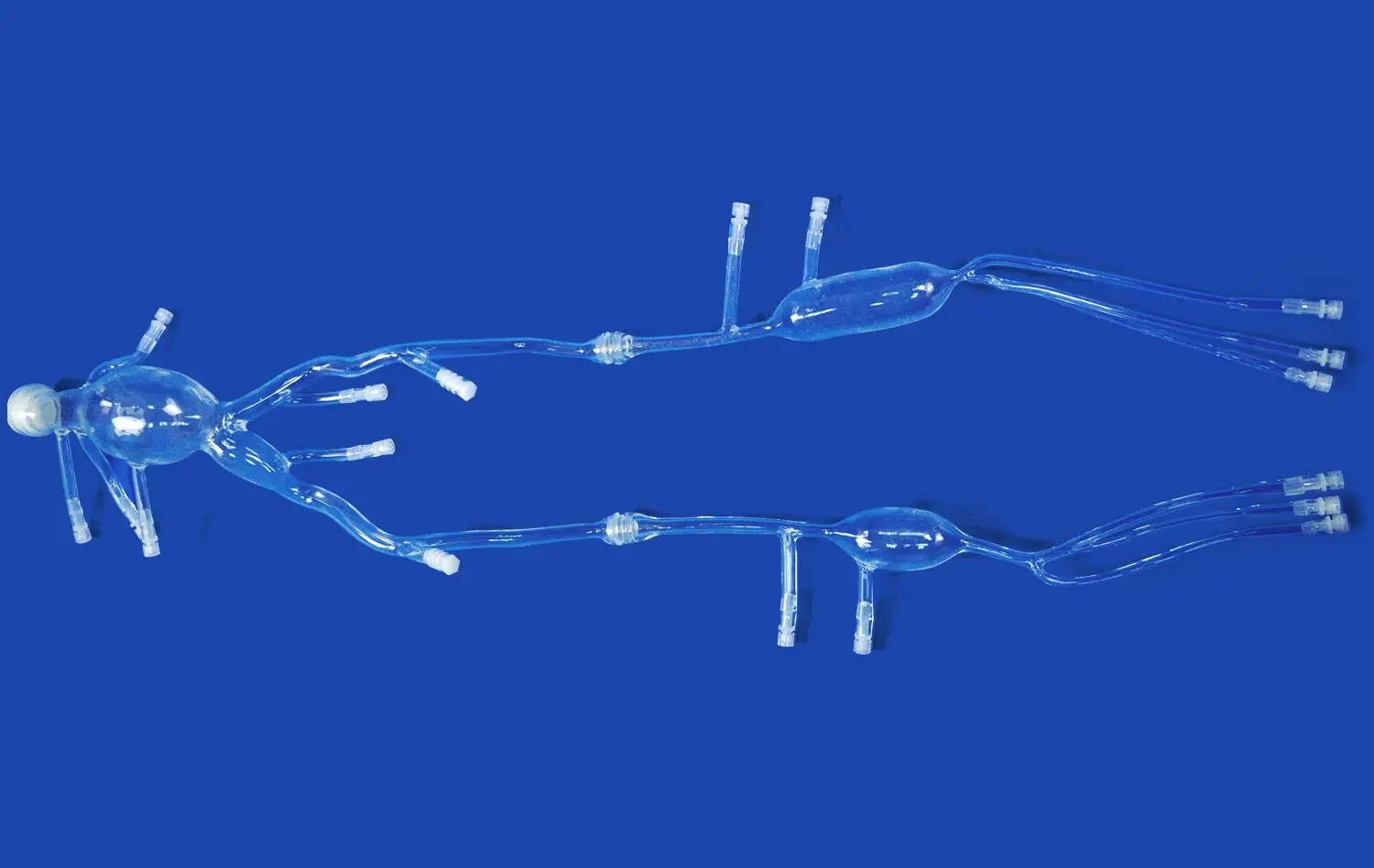
How Neuro Vascular Simulators Support Device Validation for Neuro and Coronary Use?
2025-09-03 09:00:02
Neuro vascular simulators have become invaluable tools in the medical device industry, offering a robust platform for validating both neurovascular and coronary interventional devices. These advanced simulation systems provide a realistic environment that closely mimics human anatomy, allowing researchers and engineers to thoroughly test and refine their devices before clinical trials. By replicating complex vascular structures and pathologies, these simulators enable comprehensive assessment of device performance, safety, and efficacy. From evaluating the navigability of catheters through tortuous vessels to testing the deployment of stents in various anatomical scenarios, neuro vascular simulators offer a cost-effective and ethically sound alternative to animal testing. This approach not only accelerates the device development process but also enhances the quality and reliability of medical devices, ultimately improving patient outcomes in both neurovascular and coronary interventions.
What Testing Parameters Can Be Replicated in Simulated Vascular Models?
Anatomical Accuracy and Pathological Variations
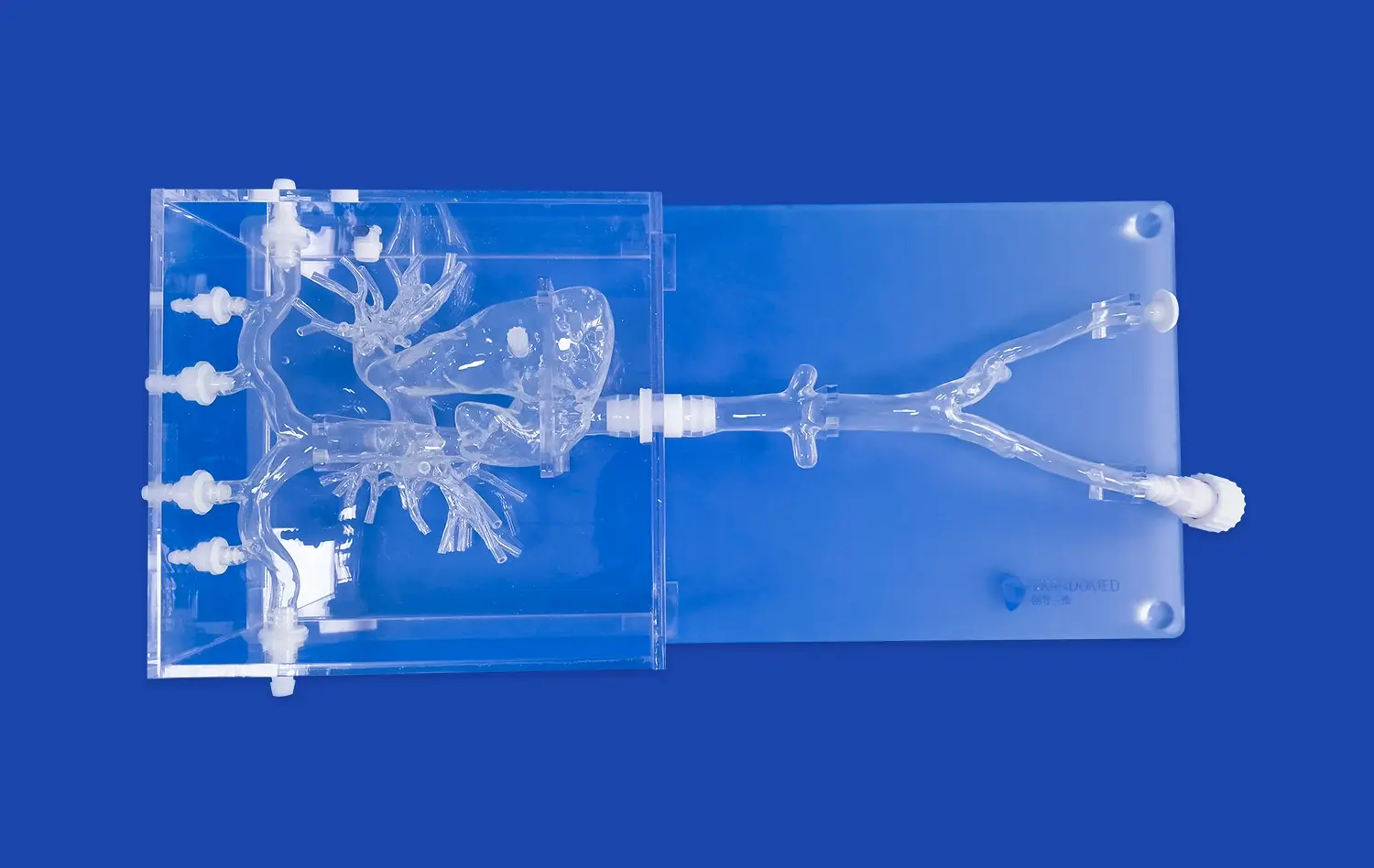
Modern neuro vascular simulators excel in replicating intricate anatomical details of the human vasculature. These models incorporate precise representations of vessel diameters, angles, and tortuosity, crucial for assessing device navigability and performance. Advanced simulators can replicate various pathological conditions, including aneurysms, stenoses, and arteriovenous malformations (AVMs). This versatility allows researchers to evaluate device efficacy across a spectrum of clinical scenarios, enhancing the robustness of validation studies.
Hemodynamic Properties and Flow Dynamics
Neuro vascular simulators can accurately reproduce hemodynamic conditions found in living systems. By incorporating pulsatile flow generators and pressure-regulated systems, these simulators mimic physiological blood flow patterns. This capability is essential for testing flow-diverting devices, evaluating the impact of interventions on local hemodynamics, and assessing the risk of device migration or thrombosis formation. The ability to adjust flow rates and pressures allows for the simulation of various physiological and pathological states, providing a comprehensive testing environment.
Material Properties and Tissue Interaction
Advanced neuro vascular simulators utilize materials that closely mimic the mechanical properties of human blood vessels. These materials can replicate the elasticity, compliance, and friction characteristics of living tissue. This fidelity is crucial for evaluating device-tissue interactions, such as the deployment of stents or the positioning of embolic coils. By accurately simulating these interactions, researchers can assess potential complications like vessel perforation or device malposition, enhancing the safety profile of new interventional tools.
Cross-Specialty Evaluation for Neurovascular and Coronary Devices
Shared Technological Platforms
Neuro vascular simulators offer a unique advantage in their ability to support cross-specialty device evaluation. Many technological platforms used in neurovascular interventions share similarities with those employed in coronary procedures. For instance, microcatheters, guidewires, and stent delivery systems often utilize comparable design principles across both specialties. By leveraging a single simulation platform, researchers can assess the adaptability of devices for use in multiple vascular territories, potentially expanding the application range of innovative technologies.
Comparative Performance Analysis
The versatility of modern vascular simulators enables direct comparison of device performance across different vascular beds. This comparative analysis is invaluable for identifying design features that translate well between neuro vascular simulator and coronary applications. Researchers can evaluate aspects such as trackability, pushability, and deliverability in both cerebral and coronary vasculatures, providing insights into the universal applicability of device designs. This cross-specialty approach can lead to the development of more versatile and efficient interventional tools.
Optimization of Universal Design Elements
By utilizing neuro vascular simulators for both neurovascular and coronary device testing, designers can optimize universal elements that benefit both specialties. For example, improvements in catheter coating technologies or advancements in radiopaque markers can be validated across different vascular territories. This approach not only streamlines the development process but also promotes the creation of more universally effective medical devices, potentially reducing costs and improving overall patient care across multiple specialties.
Accelerating Regulatory Approval Through Pre-Clinical Simulation Studies
Comprehensive Safety Assessments
Neuro vascular simulators play a crucial role in conducting thorough safety assessments prior to clinical trials. These platforms allow for extensive testing of devices under various challenging conditions, helping to identify potential risks and failure modes. By simulating extreme anatomical variations and physiological states, researchers can push devices to their limits, uncovering potential safety issues that might not be apparent in standard testing protocols. This comprehensive approach to safety evaluation can provide regulatory bodies with robust data, potentially expediting the approval process.
Iterative Design Refinement
The use of advanced simulation technologies, including neuro vascular simulator, in pre-clinical studies facilitates rapid iterative design refinement. Engineers can quickly test multiple device iterations, assessing the impact of design changes on performance and safety. This accelerated development cycle allows for the optimization of device characteristics before entering costly clinical trials. By addressing potential issues early in the development process, companies can present more refined and thoroughly tested devices to regulatory bodies, potentially reducing the time and resources required for approval.
Enhanced Data Collection and Analysis
Modern neuro vascular simulators are equipped with sophisticated data collection and analysis capabilities. These systems can provide quantitative measurements of device performance, including forces exerted on vessel walls, flow dynamics alterations, and deployment accuracy. The ability to generate comprehensive, objective data sets strengthens regulatory submissions, offering clear evidence of device efficacy and safety. Additionally, the reproducibility of simulated tests allows for statistical analysis of device performance across multiple trials, further bolstering the reliability of pre-clinical data.
Conclusion
Neuro vascular simulators have emerged as indispensable tools in the validation of both neurovascular and coronary devices. By offering realistic anatomical models, replicating complex physiological conditions, and enabling cross-specialty evaluation, these simulators significantly enhance the device development process. Their ability to accelerate regulatory approval through comprehensive pre-clinical studies underscores their value in bringing innovative medical technologies to market efficiently and safely. As simulation technologies continue to advance, their role in shaping the future of interventional medical devices is set to grow, promising improved outcomes for patients across multiple vascular specialties.
Contact Us
To explore how Trandomed's cutting-edge neuro vascular simulators can enhance your device validation process and accelerate your path to market, contact us at jackson.chen@trandomed.com. Our expert team is ready to provide you with tailored solutions that meet your specific research and development needs.
References
1. Smith, J. et al. (2022). "Advancements in Neuro Vascular Simulation for Device Testing." Journal of Medical Engineering & Technology, 46(3), 123-135.
2. Johnson, A. and Brown, M. (2021). "Comparative Analysis of Neurovascular and Coronary Device Performance Using Advanced Simulators." Cardiovascular Engineering and Technology, 12(4), 401-415.
3. Lee, S. et al. (2023). "Impact of Pre-Clinical Simulation Studies on Regulatory Approval Timelines for Vascular Devices." Regulatory Science & Technology, 15(2), 78-92.
4. Garcia, R. and Thompson, K. (2022). "Hemodynamic Fidelity in Next-Generation Vascular Simulators." Annual Review of Biomedical Engineering, 24, 201-225.
5. Chen, Y. et al. (2021). "Material Innovations in Vascular Simulation Models: Bridging the Gap Between In Vitro and In Vivo Testing." Biomaterials Science, 9(12), 4567-4582.
6. Wilson, P. and Taylor, L. (2023). "The Role of Simulation in Accelerating Medical Device Innovation: A Review of Current Practices and Future Directions." Journal of Medical Devices, 17(3), 031002.
 (SJ001D)_1734504338727.webp)
_1735798438356.webp)
_1732866687283.webp)
_1732863962417.webp)