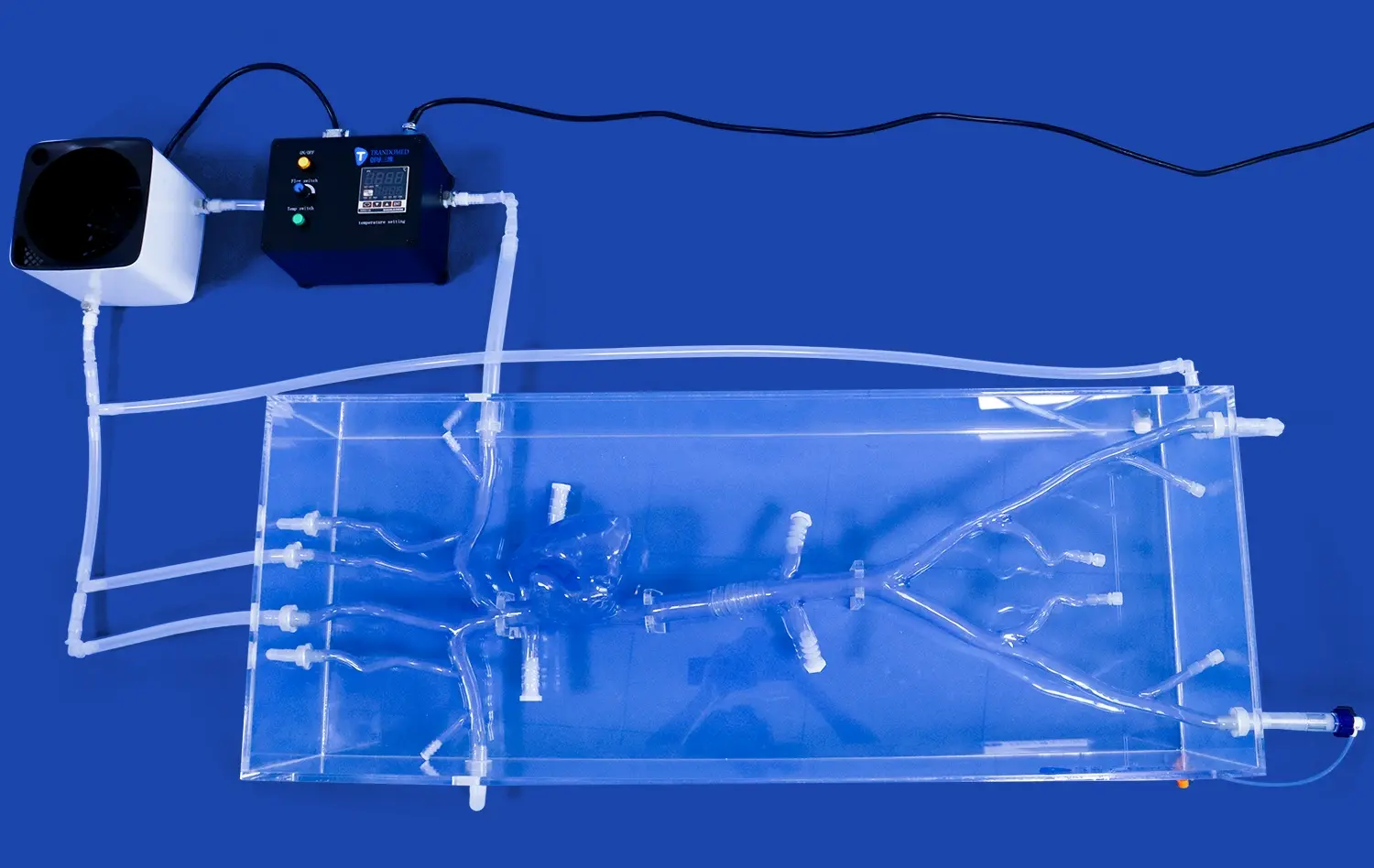
Preclinical Testing of Catheters, Stents, and Guide Wires
Anatomical Accuracy for Realistic Device Navigation
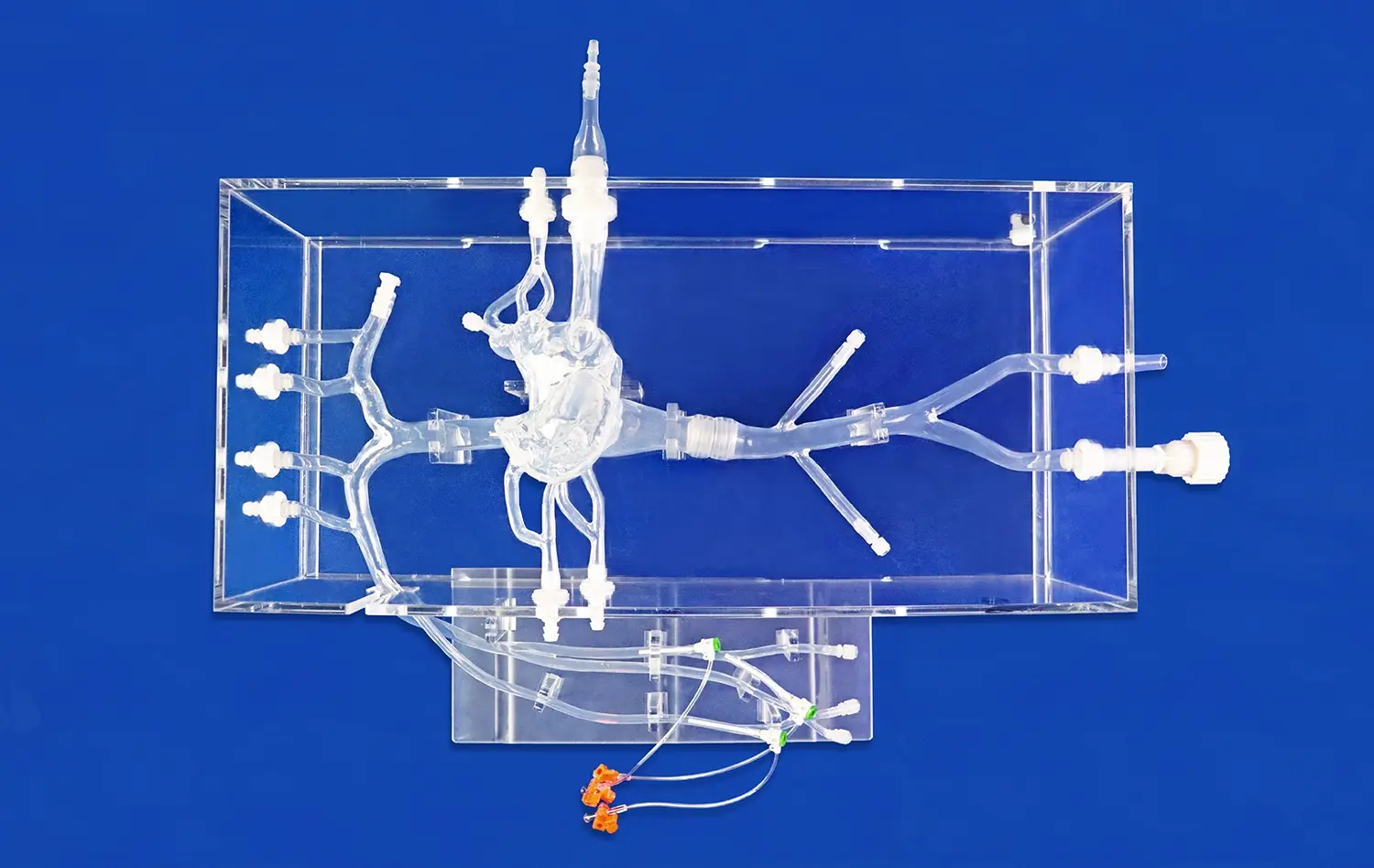
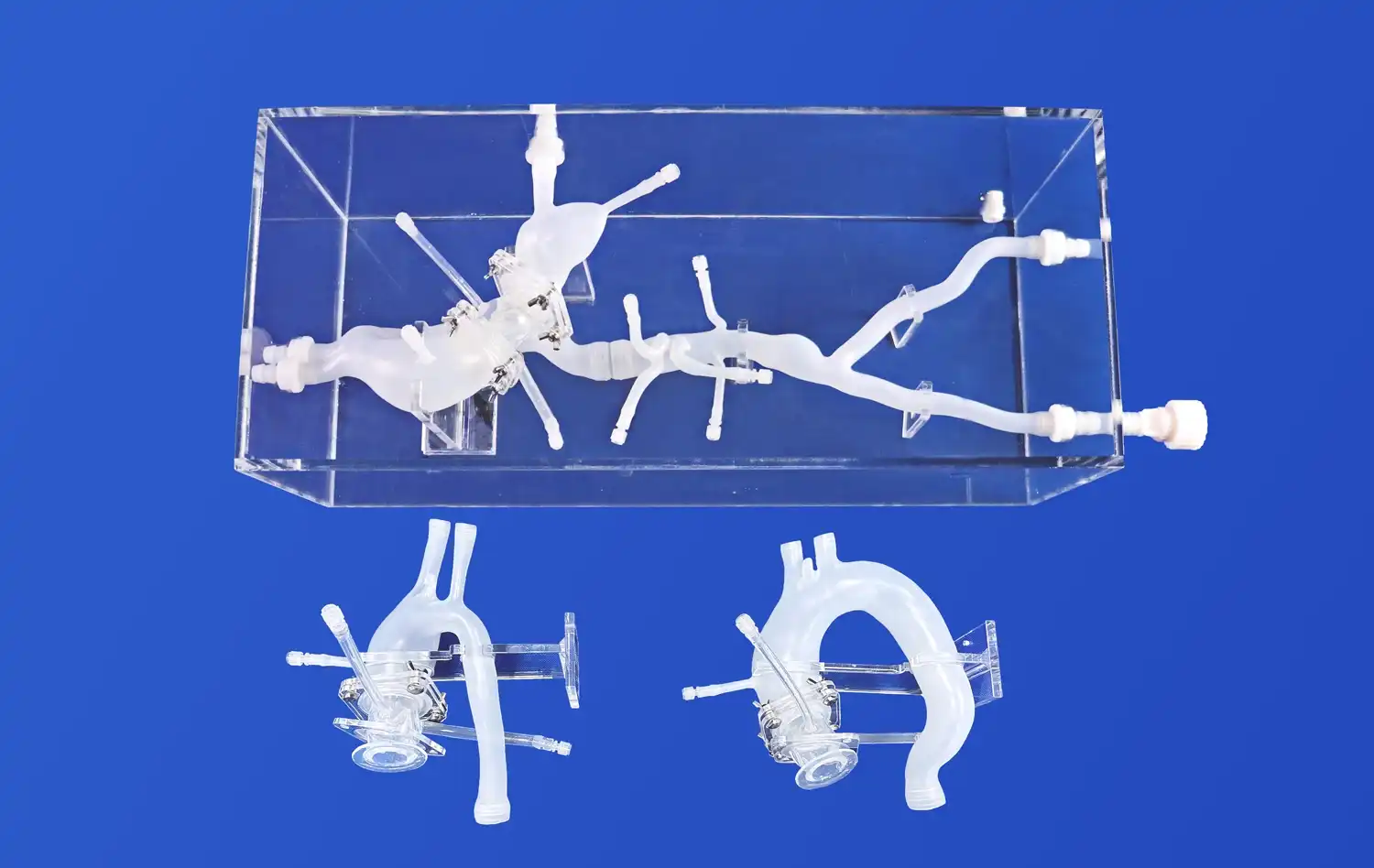
The Full Body Artery Model's anatomical precision is paramount in preclinical testing. Crafted using real human CT and MRI data, this model provides an unparalleled platform for evaluating the navigation capabilities of catheters, stents, and guide wires. The intricate vasculature, including the femoral artery, aortic arch, and cerebral vessels, allows researchers to assess device trackability and pushability in a setting that closely mimics human anatomy.
Simulating Challenging Vascular Conditions
One of the key advantages of the full body artery model is its ability to replicate complex vascular conditions. The inclusion of three intracranial aneurysms of varying sizes and a stenotic lesion in the right brain creates a challenging environment for device testing. This feature enables manufacturers to evaluate how their devices perform in difficult-to-reach areas and under pathological conditions, providing valuable insights into potential limitations or necessary design modifications.
Material Properties for Realistic Device Interaction
The silicone material used in the Full Body Artery Model, with a Shore 40A hardness, closely approximates the properties of human blood vessels. This similarity allows for a more accurate assessment of device-tissue interactions, including the potential for vessel trauma or perforation. By testing devices in a model with realistic material properties, manufacturers can better predict and mitigate risks associated with their use in clinical settings.
Reducing Procedural Risk Through Simulated Interventions
Perfecting Endovascular Techniques
The Full Body Artery Model serves as an invaluable tool for perfecting endovascular techniques. Medical professionals can practice complex procedures such as aneurysm tamponade and cerebral angiography in a risk-free environment. This hands-on experience with a high-fidelity model allows interventionalists to refine their skills, potentially reducing procedural complications and improving patient outcomes when performing actual interventions.
Device Deployment and Retrieval Scenarios
Simulating various device deployment and retrieval scenarios is crucial for ensuring procedural safety. The full body artery model allows for repeated practice of these critical steps, enabling operators to become familiar with the nuances of different devices and techniques. This familiarity can lead to smoother, more efficient procedures and reduce the risk of complications during actual patient interventions.
Identifying Potential Complications
By conducting simulated interventions on the Full Body Artery Model, researchers and clinicians can identify potential complications associated with specific devices or techniques. This proactive approach allows for the development of mitigation strategies and procedural refinements before devices are used in clinical trials or practice. The ability to anticipate and address potential issues in a controlled environment significantly enhances patient safety.
Ensuring Device Performance Before Clinical Application
Rigorous Testing Protocols
The Full Body Artery Model facilitates the implementation of rigorous testing protocols for medical devices. Manufacturers can subject their products to a wide range of simulated use cases, from routine deployments to extreme scenarios. This comprehensive testing helps ensure that devices meet or exceed performance standards and regulatory requirements before they are considered for clinical application.
Iterative Design Improvements
The insights gained from testing on the full body artery model drive iterative design improvements in medical devices. By identifying areas for enhancement in a preclinical setting, manufacturers can refine their products to better meet clinical needs and safety standards. This iterative process, facilitated by the realistic simulation environment, ultimately leads to the development of more effective and safer medical devices.
Validation of Device Compatibility
Ensuring the compatibility of different medical devices used in combination during interventional procedures is crucial for patient safety. The Full Body Artery Model allows for the validation of device compatibility in a comprehensive vascular system. This testing can reveal potential interactions or conflicts between devices, enabling manufacturers to address these issues before clinical use and providing clinicians with valuable information for procedural planning.
Conclusion
The full body artery model stands as a cornerstone in the safe validation of medical devices for vascular interventions. By providing a realistic, comprehensive platform for preclinical testing, this advanced simulator significantly reduces the risks associated with new device development and clinical application. Its ability to replicate complex anatomical structures and pathological conditions enables thorough assessment of device performance, procedural techniques, and potential complications. As medical technology continues to advance, the role of high-fidelity simulation models like the Full Body Artery Model becomes increasingly vital in ensuring patient safety and driving innovation in vascular care.
Contact Us
For those seeking to elevate their medical device validation processes, Trandomed offers state-of-the-art Full Body Artery Models that meet the highest standards of anatomical accuracy and material fidelity. As a leading 3D printed silicone medical simulators manufacturer and supplier, we provide customized solutions to meet your specific research and development needs. Experience the difference that precision-engineered simulation models can make in your product development cycle. Contact us at jackson.chen@trandomed.com to learn more about how our Full Body Artery Models can enhance your medical device validation and training programs.

_1735798438356.webp)
_1732843184544.webp)