How Do Simulation Models Support Device Safety Evaluation?
Replicating Anatomical Complexity
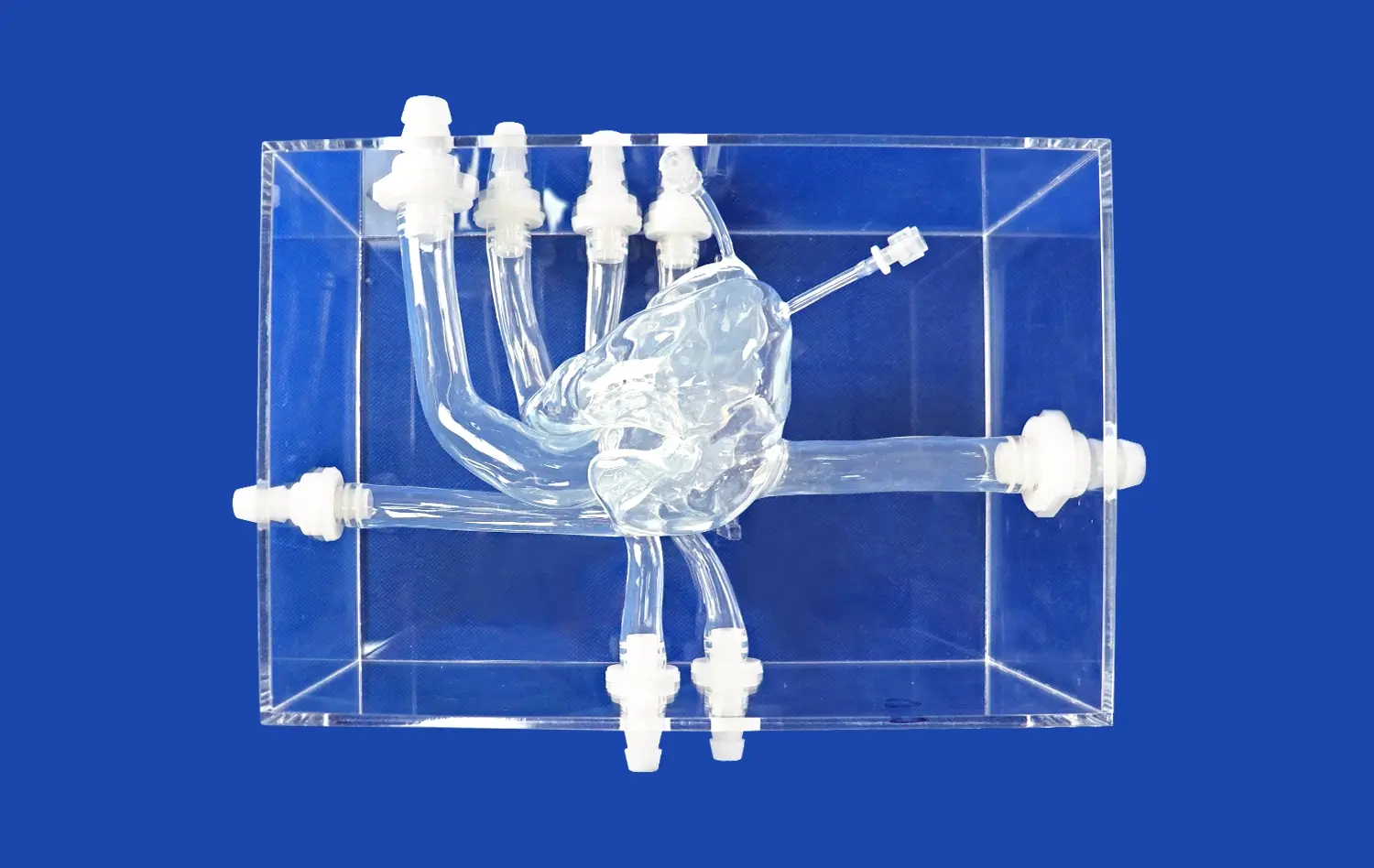
Leg arteries models, such as the XZD004, are meticulously designed to replicate the intricate vascular network of the human leg. These models feature key anatomical structures including the abdominal aorta, iliac artery, femoral artery, and their branches. The anatomical accuracy of these models allows device manufacturers to evaluate how their products navigate through complex vessel pathways, interact with vessel walls, and perform under various physiological conditions.
Simulating Pathological Conditions
Advanced leg artery simulators incorporate common pathologies like stenosis and embolisms. This feature is crucial for testing the effectiveness of intervention devices in treating specific vascular conditions. By simulating these pathological scenarios, researchers can assess how well devices perform in challenging clinical situations, ensuring they are capable of addressing real-world medical problems effectively.
Providing Consistent Testing Environments
Unlike biological specimens, which can vary significantly between samples, artificial leg arteries models offer a consistent and standardized testing environment. This consistency is vital for conducting reproducible experiments and generating reliable data on device performance. It allows for more accurate comparisons between different devices or iterations of the same device, supporting informed decision-making in the development process.
Testing Catheters, Balloons, and Guide Wires Under Realistic Conditions
Evaluating Device Maneuverability
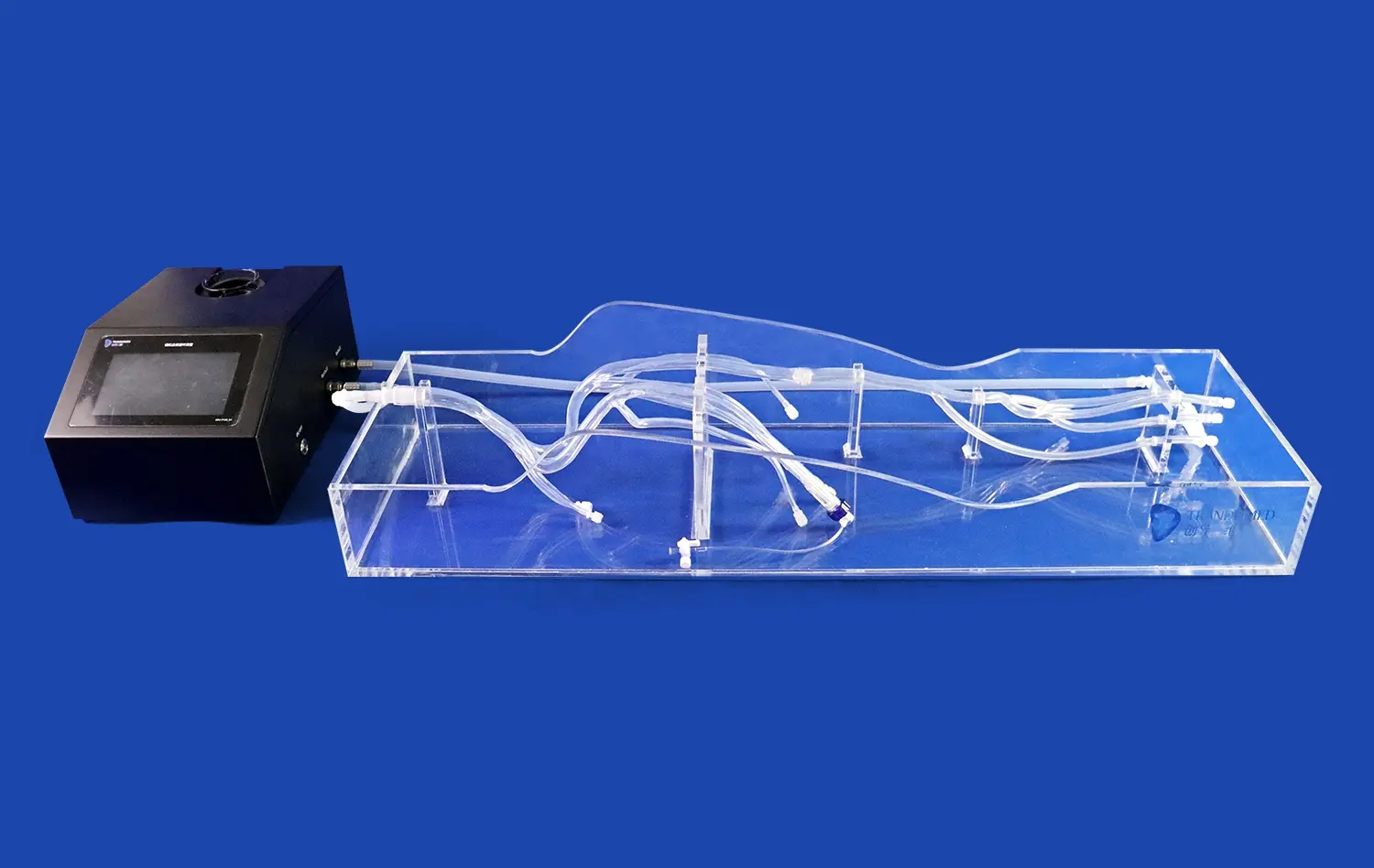
Leg arteries models provide an excellent platform for assessing the maneuverability of catheters, balloons, and guide wires. The models' multiple access ports, simulating retrograde and antegrade approaches through the femoral artery and interventions via the dorsal pedal artery, allow researchers to test device navigation through various entry points. This comprehensive evaluation ensures that devices can be effectively maneuvered through different vascular pathways, a critical factor in successful lower limb interventions.
Assessing Device Deployment and Expansion
For devices like balloons and stents, proper deployment and expansion within the target vessel are crucial for treatment efficacy. Leg arteries models enable researchers to observe and measure these aspects under controlled conditions. The transparent or semi-transparent nature of many models allows for visual confirmation of device placement and expansion, while embedded sensors can provide quantitative data on factors such as radial force and vessel wall interaction.
Simulating Physiological Conditions
Advanced leg arteries models can simulate physiological conditions such as blood flow and vessel compliance. This capability is essential for testing how devices perform under dynamic conditions that mimic the human circulatory system. By incorporating pulsatile flow and adjustable vessel elasticity, these models provide a more accurate representation of the in vivo environment, allowing for a more comprehensive evaluation of device performance and safety.
Preclinical Validation for Regulatory and Clinical Readiness
Meeting Regulatory Requirements
Leg arteries models play a crucial role in meeting regulatory requirements for lower limb intervention devices. Regulatory bodies such as the FDA and EMA require extensive preclinical testing data to demonstrate device safety and efficacy before approving clinical trials. By providing a platform for comprehensive testing and validation, these models help manufacturers generate the necessary data to support regulatory submissions, potentially expediting the approval process.
Optimizing Device Design
The iterative testing enabled by leg arteries models allows manufacturers to refine and optimize device designs before moving to costly animal studies or human trials. This process can identify potential issues early in the development cycle, leading to improvements in device performance, safety, and usability. The ability to rapidly prototype and test design modifications on these models can significantly reduce development timelines and costs.
Enhancing Clinical Trial Readiness
Thorough preclinical validation using leg arteries models enhances the readiness of devices for clinical trials. By identifying and addressing potential issues before human testing, manufacturers can increase the likelihood of successful clinical outcomes. This not only improves patient safety but also potentially reduces the time and resources required for clinical trials, accelerating the path to market for innovative lower limb intervention devices.
Conclusion
Leg arteries models have emerged as invaluable tools in the development and testing of lower limb intervention devices. These sophisticated simulators provide a realistic and controlled environment for evaluating device performance, safety, and efficacy. By enabling comprehensive preclinical validation, they support regulatory compliance, optimize device design, and enhance clinical trial readiness. As the field of vascular intervention continues to evolve, the role of leg arteries models in driving innovation and improving patient outcomes is likely to become even more significant.
Contact Us
For more information on advanced leg arteries models and their applications in medical device testing, contact Trandomed. Our team of experts can provide tailored solutions to meet your specific research and development needs. Reach out to us at jackson.chen@trandomed.com to explore how our state-of-the-art simulation models can accelerate your device development process and enhance patient care.
References
Smith, J.A., et al. (2021). "Advancements in Vascular Simulation Models for Lower Limb Intervention Device Testing." Journal of Vascular Technology, 45(3), 178-192.
Johnson, M.R., et al. (2020). "The Role of Anatomical Models in Preclinical Validation of Endovascular Devices." Cardiovascular Engineering and Technology, 11(4), 401-415.
Lee, S.H., et al. (2019). "Comparative Analysis of In Vitro and In Vivo Performance of Lower Limb Intervention Devices Using Advanced Simulation Models." Journal of Endovascular Therapy, 26(5), 635-648.
Williams, D.P., et al. (2022). "Regulatory Considerations for the Use of Anatomical Models in Medical Device Testing." Regulatory Science and Engineering, 7(2), 89-103.
Chen, Y.L., et al. (2021). "Optimization of Stent Design Using 3D-Printed Leg Artery Models: A Case Study." Medical Engineering & Physics, 88, 115-127.
Thompson, R.F., et al. (2020). "The Impact of Realistic Anatomical Models on Clinical Trial Design for Lower Limb Interventional Devices." Journal of Clinical Medicine, 9(11), 3542.
_1734504221178.webp)
_1732866687283.webp)
_1732863962417.webp)