How Are Mitral Valve Models Used for Device Compatibility Assessment?
Anatomical Accuracy and Structural Evaluation
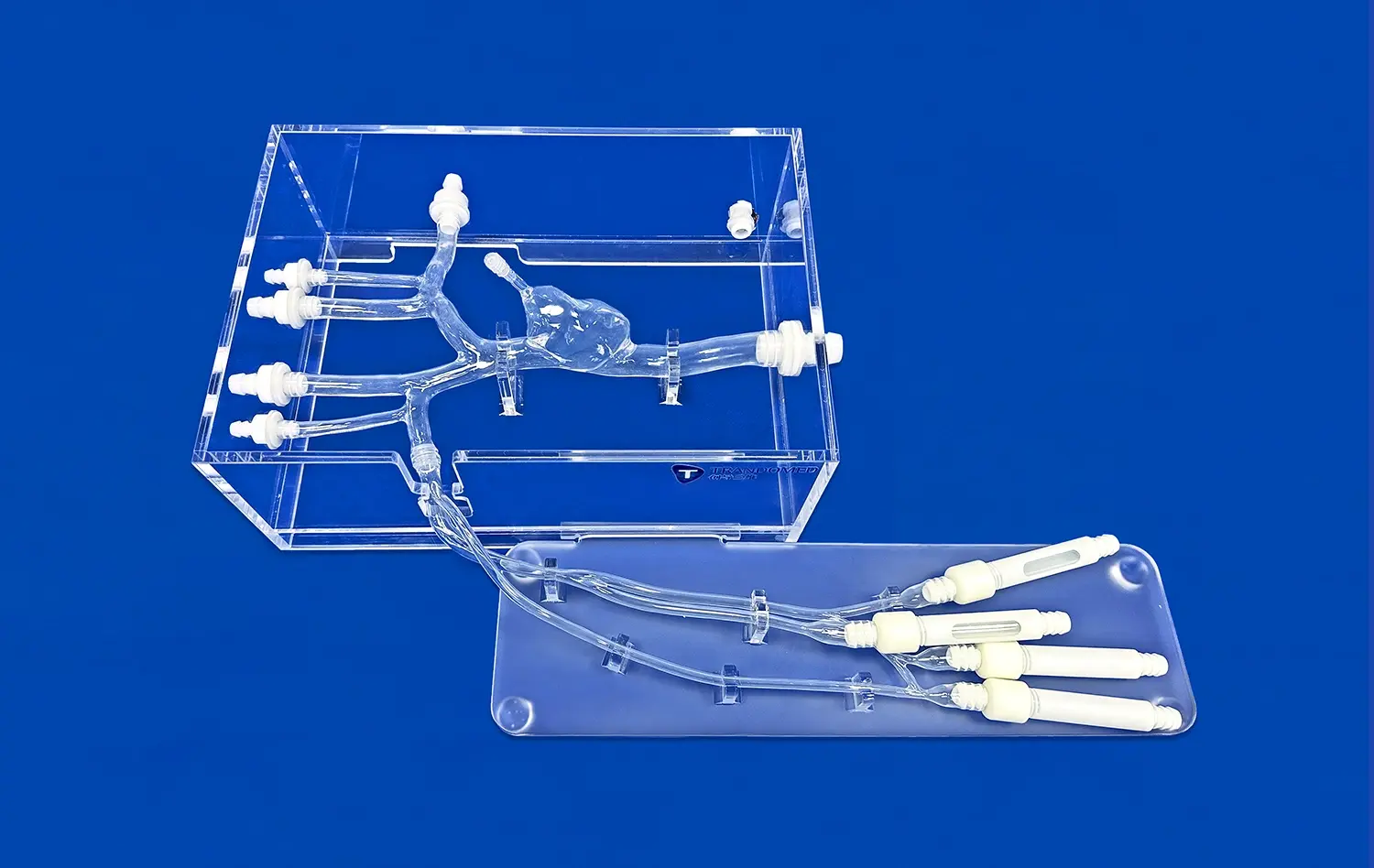
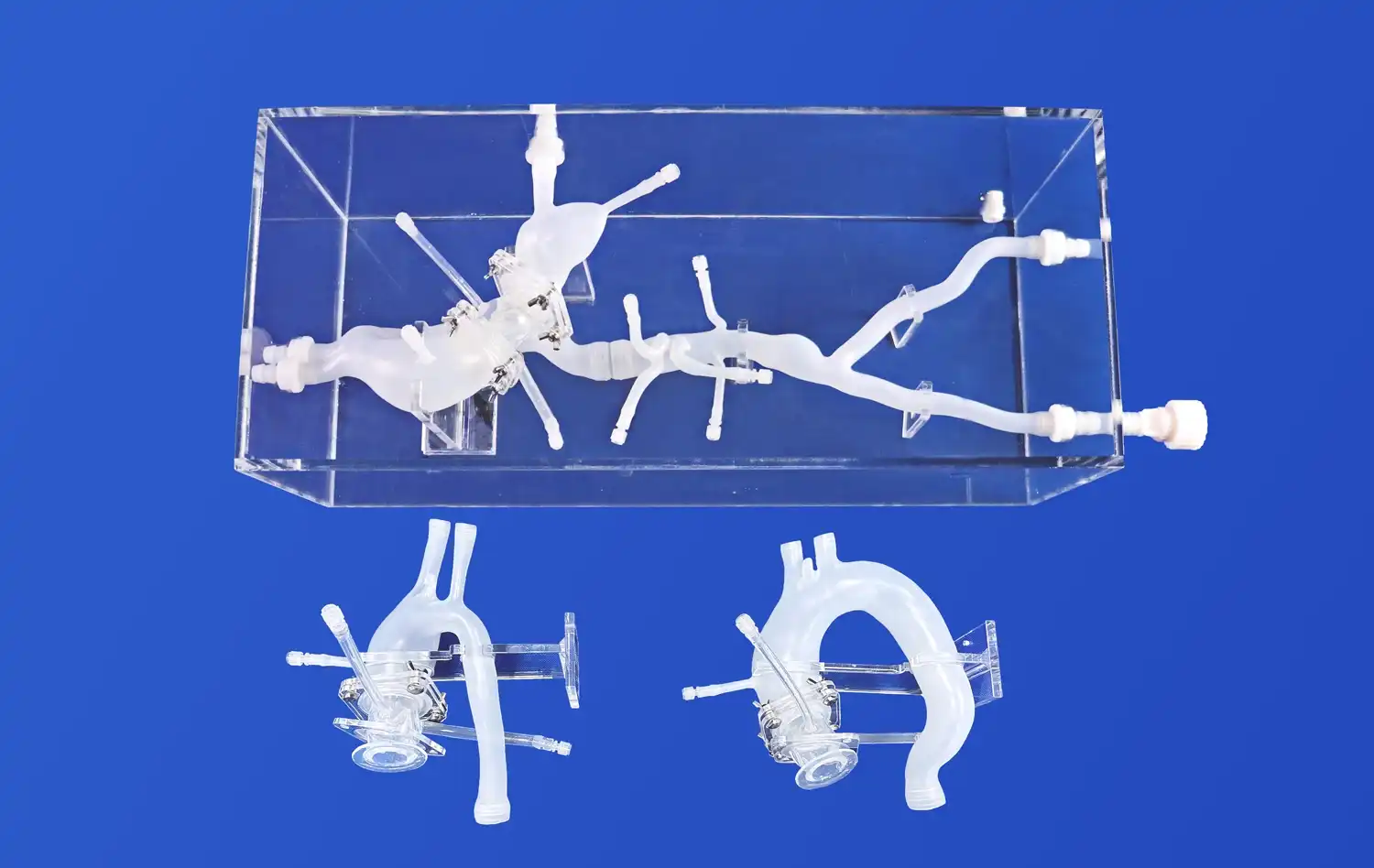
Mitral valve models, such as the Mitral Valve Model (XXD006) offered by Trandomed, provide an anatomically accurate representation of the heart's structure. These models extend from the femoral vein to the right heart, encompassing key vascular structures like the femoral vein, iliac veins, inferior vena cava (IVC), right and left atrium, left ventricle, mitral valve, and superior vena cava (SVC). This comprehensive design allows researchers to evaluate the physical compatibility of cardiovascular devices with the surrounding cardiac anatomy.The intricate detailing of these models, including the mitral valve leaflets, chordae tendineae, and papillary muscles, enables a thorough assessment of how devices interact with these delicate structures. This level of precision is crucial for identifying potential issues such as interference with valve movement, unintended tissue damage, or improper device positioning.
Dynamic Functionality Testing
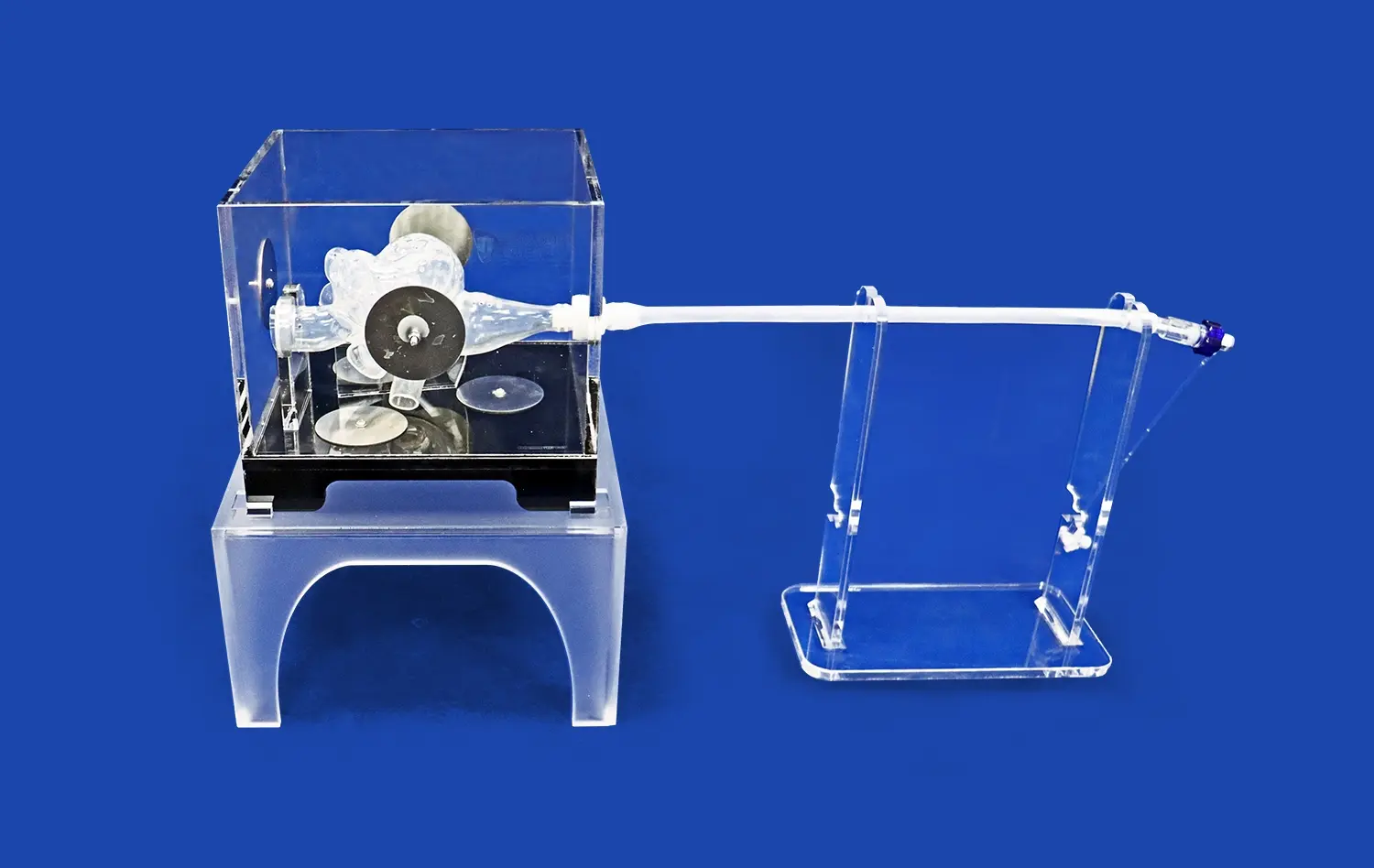
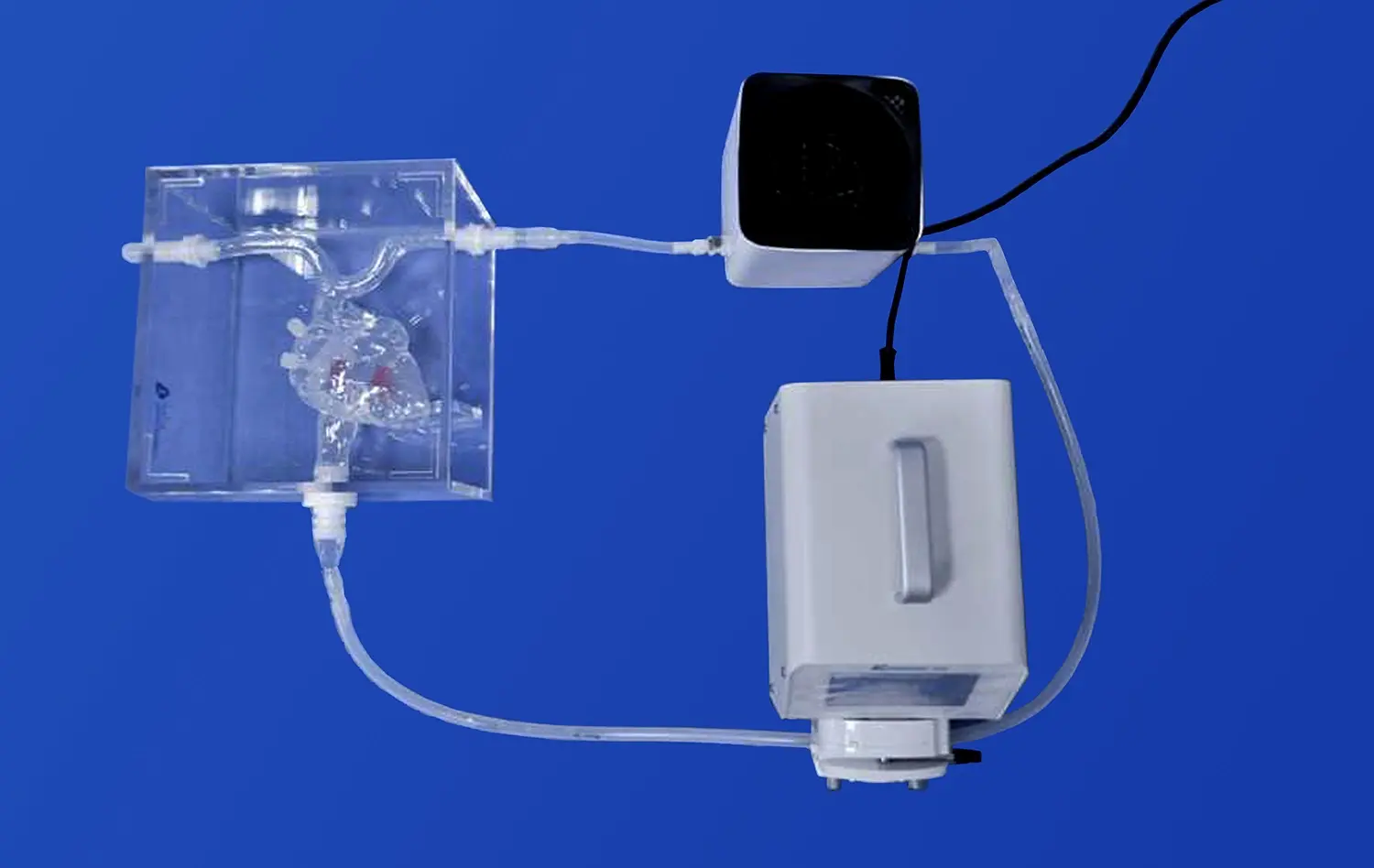
Advanced mitral valve models incorporate dynamic functionality, simulating the opening and closing of the valve under various hemodynamic conditions. When connected to systems like the EDU-heart pump, these models can replicate the pulsatile flow of blood through the heart chambers. This dynamic capability is essential for testing the performance of devices such as mitral valve repair clips or transcatheter mitral valve replacements under realistic physiological conditions.By observing how devices interact with the moving components of the mitral valve, researchers can assess factors like:
- Valve coaptation and leaflet mobility post-device implantation
- Potential for device migration or dislodgement during cardiac cycles
- Impact on blood flow patterns and potential for thrombus formation
Material Interaction Studies
The choice of materials in cardiovascular device design is critical for ensuring biocompatibility and long-term durability. Mitral valve models constructed from materials like Silicone Shore 40A, as used in Trandomed's models, provide a realistic substrate for evaluating material interactions. This allows researchers to:
- Assess the frictional properties between device materials and valve tissues
- Study potential wear patterns or material degradation over simulated time periods
- Evaluate the ease of device deployment and retrieval in a realistic anatomical setting.
These material interaction studies are invaluable for refining device designs and selecting optimal materials for specific cardiac interventions.
Simulating Clinical Scenarios for Cardiovascular Device Evaluation
Pathological Condition Replication
One of the most significant advantages of advanced mitral valve models is their ability to replicate various pathological conditions. For instance, models can be customized to simulate:
- Mitral valve prolapse with specific leaflet involvement (e.g., P2 segment)
- Chordal rupture or elongation
- Annular dilatation
- Calcification patterns.
These pathological replications allow device manufacturers to test their products under diverse clinical scenarios, ensuring efficacy across a spectrum of mitral valve diseases. The ability to simulate specific patient anatomies based on CT or MRI data further enhances the clinical relevance of these evaluations.
Procedural Simulation and Training
Mitral valve models serve as excellent platforms for procedural simulation and training. They allow clinicians to practice complex interventions such as:
- Transcatheter mitral valve replacement (TMVR)
- Edge-to-edge repair techniques
- Annuloplasty procedures
- Chordal replacement or repair.
These simulations not only aid in device evaluation but also contribute to the training and skill development of interventional cardiologists and cardiac surgeons. The realistic feel and response of high-quality silicone models provide an immersive training experience that closely mimics real-world scenarios.
Complication Management and Risk Assessment
Cardiovascular device testing on mitral valve models enables researchers to anticipate and assess potential complications. By simulating various anatomical variations and procedural challenges, teams can:
- Identify risks of left ventricular outflow tract obstruction post-TMVR
- Evaluate the potential for paravalvular leaks
- Assess the risk of coronary artery compression
- Study the impact of device deployment on surrounding structures.
This proactive approach to risk assessment is crucial for developing safer devices and refining implantation techniques, ultimately leading to improved patient outcomes.
Accelerating Device Development Through Model-Based Testing
Rapid Prototyping and Iteration
The use of mitral valve models significantly accelerates the device development process by enabling rapid prototyping and iteration. Manufacturers can quickly test design modifications on these models, observing their impact on device performance and interaction with valve anatomy. This iterative process allows for:
- Fine-tuning of device dimensions and geometries
- Optimization of deployment mechanisms
- Refinement of materials and surface properties.
The ability to conduct multiple iterations in a controlled, reproducible environment streamlines the development pipeline, potentially reducing time-to-market for innovative cardiovascular devices.
Comparative Analysis and Benchmarking
Mitral valve models provide a standardized platform for comparative analysis and benchmarking of different devices or design iterations. This allows researchers to:
- Evaluate the relative performance of competing device designs
- Assess improvements in new device generations against predecessors
- Compare novel approaches with established treatment modalities.
Such comparative studies are invaluable for making informed decisions during the device development process and for demonstrating the potential benefits of new technologies to regulatory bodies and healthcare providers.
Predictive Modeling and Long-Term Performance Assessment
Advanced mitral valve models, especially when combined with computational simulations, enable predictive modeling of long-term device performance. By subjecting devices to accelerated wear testing or simulating years of cardiac cycles, researchers can:
- Predict potential failure modes and device longevity
- Assess the impact of chronic stresses on device materials and structures
- Evaluate the long-term effects on valve function and cardiac hemodynamics.
These predictive capabilities are crucial for developing devices with improved durability and for providing valuable data to support regulatory submissions and clinical adoption.
Conclusion
Mitral valve models have revolutionized cardiovascular device testing, offering a versatile and reliable platform for assessing device compatibility, simulating clinical scenarios, and accelerating development processes. From anatomical accuracy to dynamic functionality, these models provide invaluable insights that drive innovation in cardiac care. As technology advances, the integration of 3D printing, smart materials, and computational modeling promises to further enhance the capabilities of mitral valve models, paving the way for more effective, safer, and patient-specific cardiovascular devices. The continued refinement and application of these models will undoubtedly play a pivotal role in shaping the future of cardiac interventions and improving outcomes for patients with mitral valve disease.
Contact Us
For cutting-edge mitral valve models that can elevate your cardiovascular device testing, look no further than Trandomed. Our high-precision, customizable models offer unparalleled anatomical accuracy and dynamic functionality. Experience the benefits of accelerated development, enhanced training capabilities, and improved device performance. Contact us at jackson.chen@trandomed.com to explore how our advanced mitral valve models can revolutionize your cardiovascular research and development efforts.
_1734507205192.webp)