How Can Replaceable Models Validate Valve System Functionality?
Anatomical Accuracy and Customization
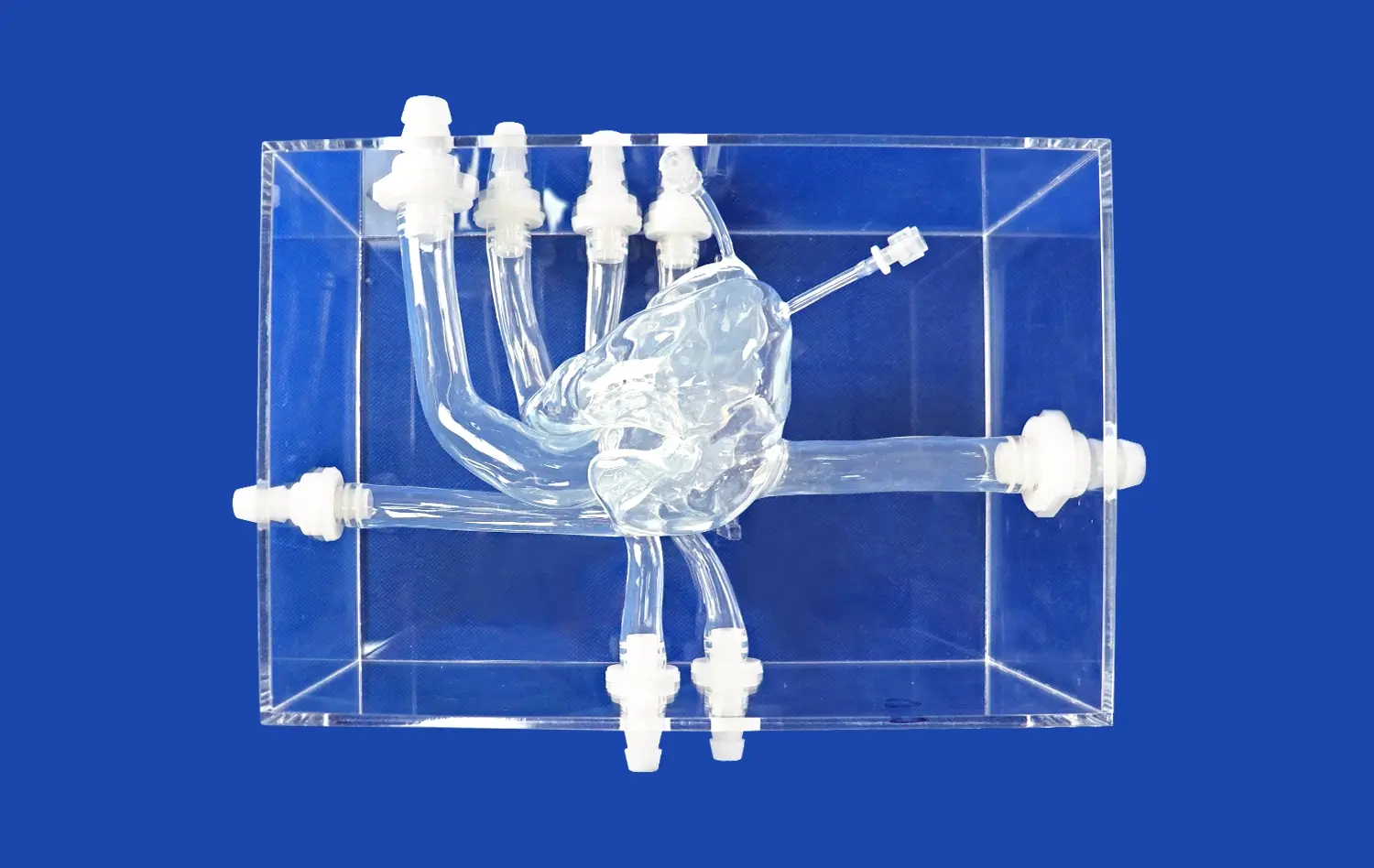
Aortic arch replaceable models provide an unparalleled level of anatomical accuracy, crucial for validating valve system functionality. These models are constructed using advanced 3D printing techniques based on real patient data from CT and MRI scans. This approach ensures that the models replicate the intricate details of the aortic arch, including its curvature, branch vessels, and surrounding structures.
The customizable nature of these models allows researchers to simulate various anatomical variations and pathological conditions. For instance, Trandomed's aortic arch replaceable model can be tailored to represent different arch types (Type I, II, or III) or even abnormal arches with aortic malformations. This versatility enables comprehensive testing of valve systems across a wide range of anatomical scenarios, ensuring their functionality in diverse patient populations.
Multi-functional Design for Comprehensive Testing
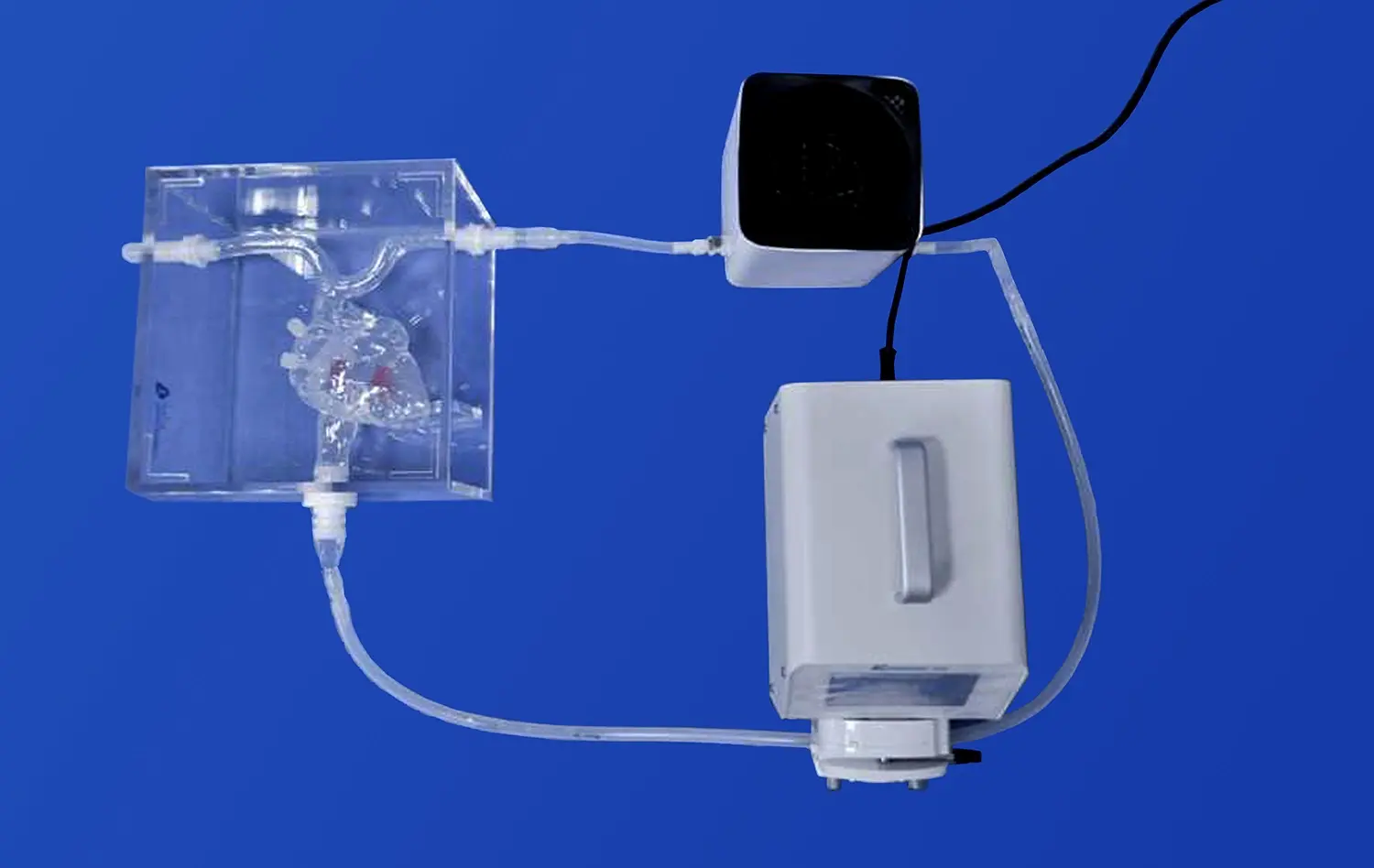
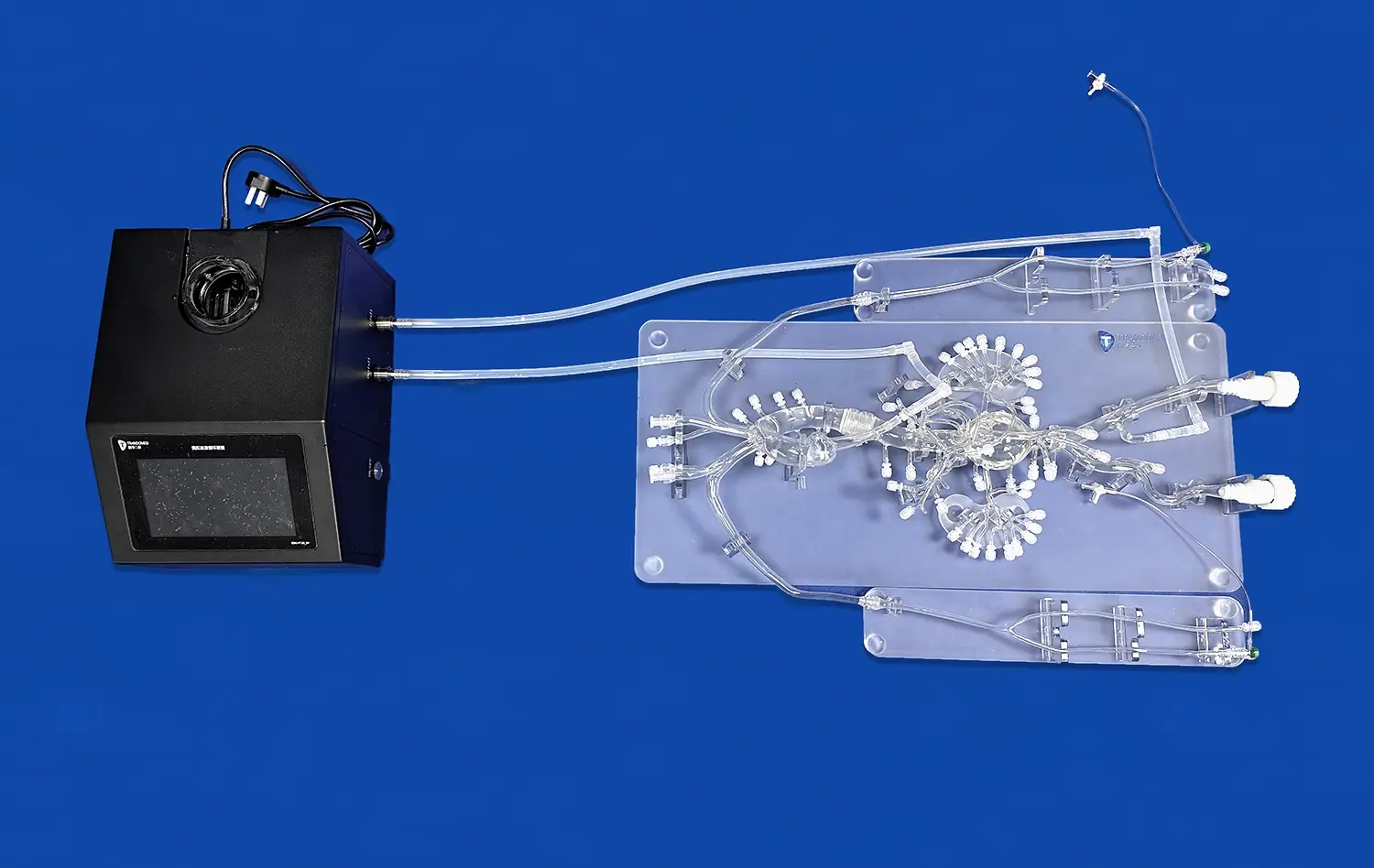
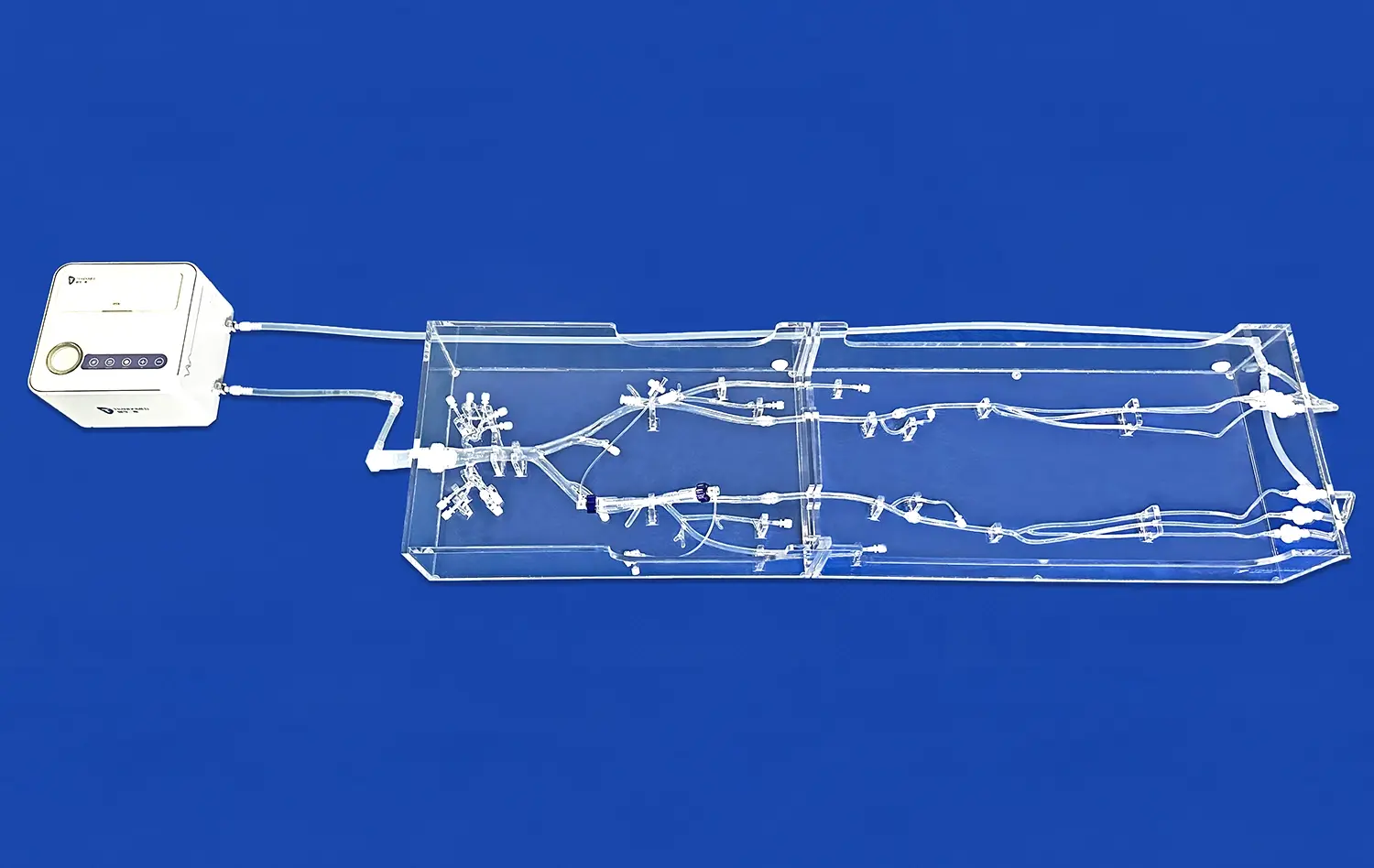
The multi-functional design of aortic arch replaceable models facilitates comprehensive testing of transcatheter aortic valve systems. These models typically comprise multiple interconnected components, such as the left ventricle, thoracic aorta, and abdominal aorta. The modular nature of these components, often connected via transparent pagoda connectors, allows for easy assembly and disassembly, providing flexibility in testing various aspects of valve system functionality.
Researchers can assess valve deployment, positioning, and sealing within the aortic root, as well as evaluate the delivery system's navigation through the aortic arch. The ability to interchange different components also enables the simulation of various pathological conditions, such as calcified aortic valves or stenotic vessels, further enhancing the validity of functional testing.
Material Properties and Hemodynamic Simulation
The material properties of aortic arch replaceable models play a crucial role in validating valve system functionality. High-quality silicone materials, such as those used by Trandomed (Shore 40A), closely mimic the mechanical properties of human vasculature. This similarity allows for realistic assessment of valve expansion, anchoring, and interaction with the aortic wall.
Moreover, these models can be integrated into flow loops to simulate physiological hemodynamics. By incorporating pulsatile flow and pressure conditions, researchers can evaluate the valve's performance under realistic conditions, including its ability to maintain proper coaptation, minimize paravalvular leakage, and withstand the dynamic forces experienced during the cardiac cycle.
Key Metrics for Transcatheter Aortic Valve Performance Evaluation
Deployment Accuracy and Positioning
One of the primary metrics in evaluating transcatheter aortic valve performance is deployment accuracy and positioning. Aortic arch replaceable models allow researchers to assess the precision with which a valve can be deployed at the target site. This includes evaluating the ability to achieve proper alignment with the native annulus, avoiding coronary ostia obstruction, and maintaining an appropriate depth of implantation.
Researchers can use various imaging modalities, such as fluoroscopy or echocardiography, in conjunction with these models to quantify deployment accuracy. Metrics such as the degree of paravalvular gap, valve frame expansion symmetry, and the distance from key anatomical landmarks can be measured and analyzed to optimize valve design and deployment techniques.
Hemodynamic Performance
Hemodynamic performance is a critical aspect of transcatheter aortic valve evaluation. Aortic arch replaceable models integrated into flow loops enable the assessment of various hemodynamic parameters. These include:
- Transvalvular pressure gradient: Measuring the pressure difference across the valve during systole and diastole.
- Effective orifice area: Calculating the functional opening of the valve to assess its ability to facilitate blood flow.
- Regurgitation fraction: Quantifying the amount of retrograde flow through the valve during diastole.
- Paravalvular leakage: Assessing the presence and severity of leaks around the valve frame.
By analyzing these metrics, researchers can optimize valve designs to minimize pressure gradients, maximize effective orifice area, and reduce regurgitation and paravalvular leakage.Durability and Fatigue Resistance
Long-term durability is essential for transcatheter aortic valves, and aortic arch replaceable models play a crucial role in evaluating this aspect. These models can be incorporated into accelerated wear testers that simulate years of cardiac cycles in a compressed timeframe. Key metrics for durability assessment include:
- Leaflet integrity: Monitoring for signs of tearing, calcification, or other structural changes.
- Frame stability: Assessing the valve frame for fractures, deformation, or migration.
- Suture integrity: Evaluating the durability of connections between components.
- Hemodynamic stability: Tracking changes in valve performance over time.
By subjecting valve systems to millions of cycles under physiological conditions, researchers can identify potential failure modes and optimize designs for long-term durability.Reducing Clinical Risk Through Preclinical Simulation
Procedural Refinement and Complication Mitigation
Aortic arch replaceable models serve as invaluable tools for reducing clinical risk by enabling thorough preclinical simulation. These models allow clinicians and device manufacturers to refine procedural techniques and anticipate potential complications before human trials. By simulating various anatomical challenges, such as heavily calcified vessels or tortuous aortic arches, teams can develop strategies to navigate difficult cases safely.
Moreover, these models facilitate the simulation of rare but serious complications, such as valve embolization or coronary obstruction. By practicing management techniques for these scenarios, clinicians can enhance their preparedness and response times, potentially reducing patient risk during actual procedures.
Device Iteration and Optimization
The use of aortic arch replaceable models in preclinical testing allows for rapid iteration and optimization of transcatheter aortic valve systems. Manufacturers can evaluate multiple design variations quickly and cost-effectively, identifying the most promising configurations for further development. This iterative process, conducted in a risk-free environment, can lead to significant improvements in device performance and safety before progressing to clinical trials.
For instance, researchers can experiment with different frame designs, leaflet materials, or delivery system components to enhance valve durability, ease of deployment, or compatibility with challenging anatomies. The ability to test these iterations in anatomically accurate models reduces the likelihood of unforeseen issues arising during human trials.
Training and Skill Development
Aortic arch replaceable models play a crucial role in training interventional cardiologists and surgical teams, thereby reducing clinical risk. These models provide a realistic platform for hands-on practice of TAVR procedures, allowing clinicians to familiarize themselves with new devices and techniques without putting patients at risk. The ability to repeat procedures, practice rare scenarios, and receive immediate feedback contributes to skill development and procedural confidence.
Furthermore, these models can be used in team-based simulation exercises, improving communication and coordination among different members of the heart team. By rehearsing complex cases and emergency scenarios, teams can enhance their collective performance and decision-making abilities, ultimately leading to improved patient outcomes in real clinical settings.
Conclusion
Aortic arch replaceable models have emerged as indispensable tools in the performance testing of transcatheter aortic valve systems. These advanced silicone simulators offer unparalleled anatomical accuracy, customization options, and versatility in evaluating valve functionality, hemodynamic performance, and long-term durability. By enabling comprehensive preclinical testing, procedural refinement, and clinician training, these models significantly contribute to reducing clinical risk and improving patient outcomes in TAVR procedures. As the field of structural heart interventions continues to evolve, the role of these sophisticated models in driving innovation and enhancing safety will undoubtedly expand, shaping the future of cardiovascular device development and clinical practice.
Contact Us
Elevate your transcatheter aortic valve system testing and development with Trandomed's state-of-the-art aortic arch replaceable models. Our customizable, high-fidelity simulators offer unparalleled anatomical accuracy and versatility, enabling comprehensive performance evaluation and risk reduction. Experience the benefits of advanced preclinical testing and unlock new possibilities in cardiovascular device innovation. Contact us today at jackson.chen@trandomed.com to explore how our cutting-edge models can accelerate your research and development efforts.
_1734504221178.webp)
_1734507815464.webp)