The Importance of Leg Arteries Models in Vascular Device Innovation
2025-08-05 09:00:01
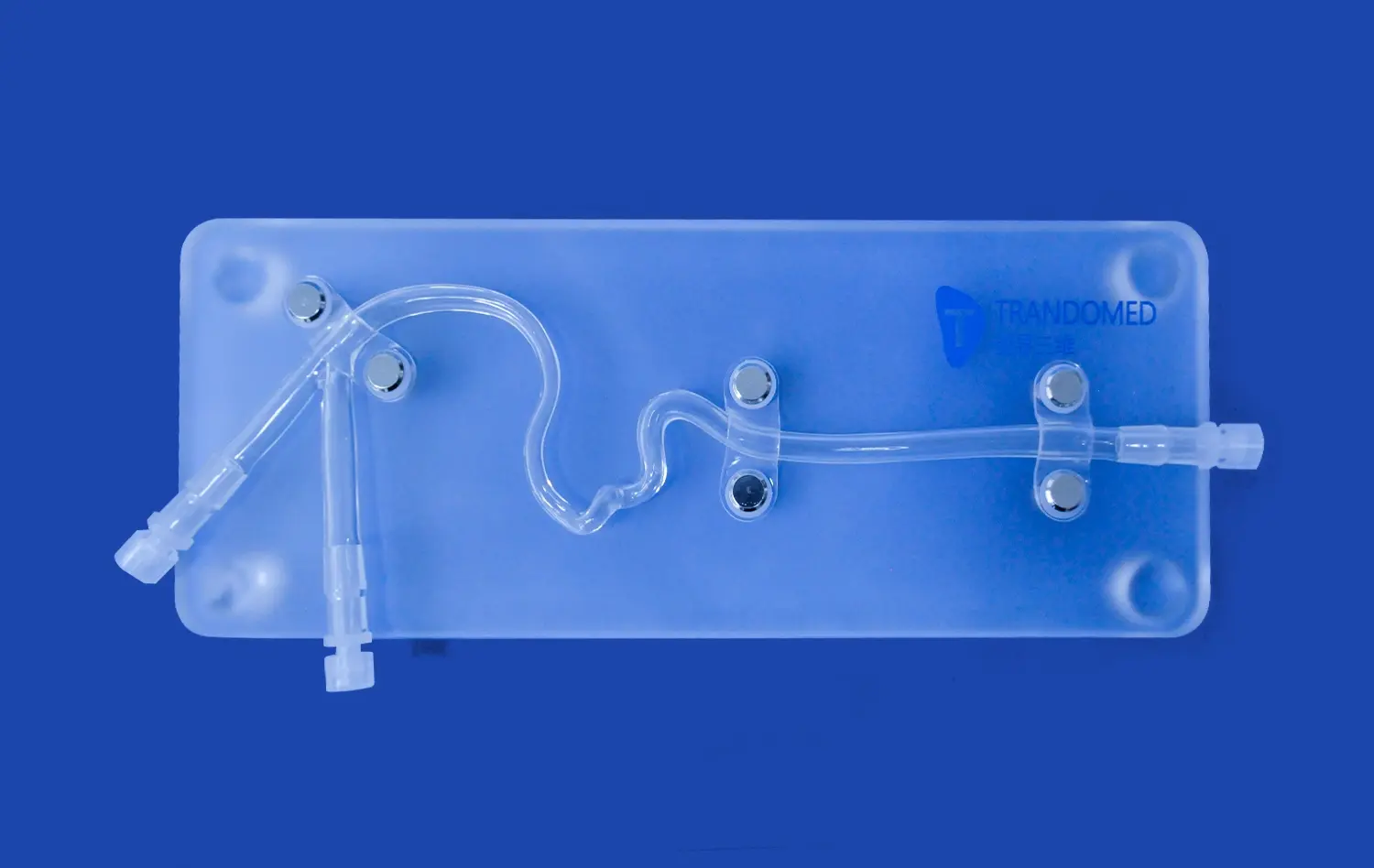
Leg arteries models play a crucial role in advancing vascular device innovation, offering a realistic and safe environment for researchers, engineers, and medical professionals to develop and refine new technologies. These sophisticated replicas of the human leg's vascular system, such as the Leg Arteries Model (XZD004), provide an invaluable platform for testing and improving interventional devices, surgical techniques, and treatment strategies. By accurately simulating the complex arterial network, including common pathologies like stenosis and embolisms, these models accelerate research, optimize design processes, and facilitate early-stage validation. This ultimately leads to more effective and safer vascular devices, reduced development costs, and improved patient outcomes in the treatment of peripheral artery disease and related conditions.
How Do Models Accelerate Research and Product Iteration?
Realistic Simulation of Vascular Anatomy
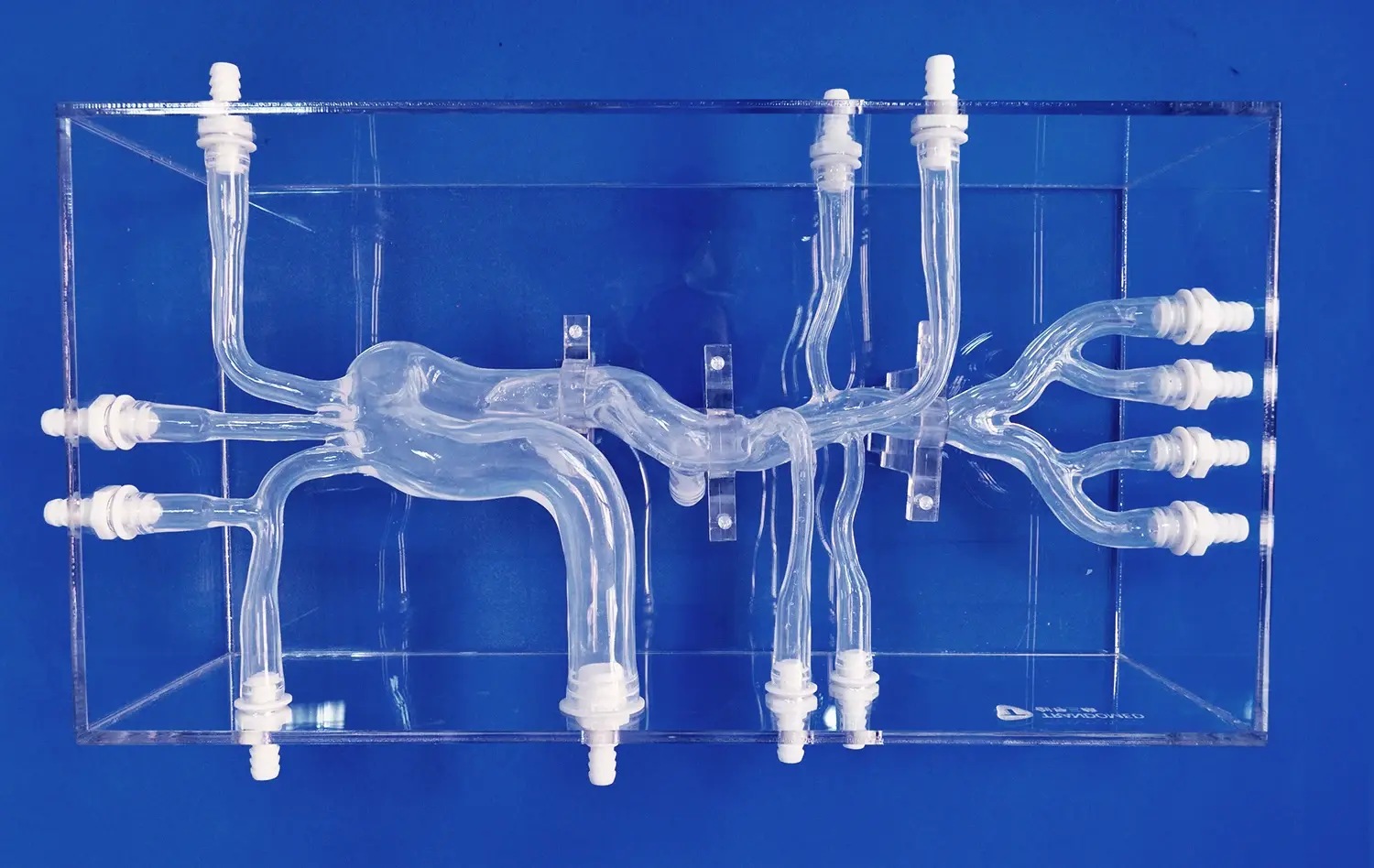
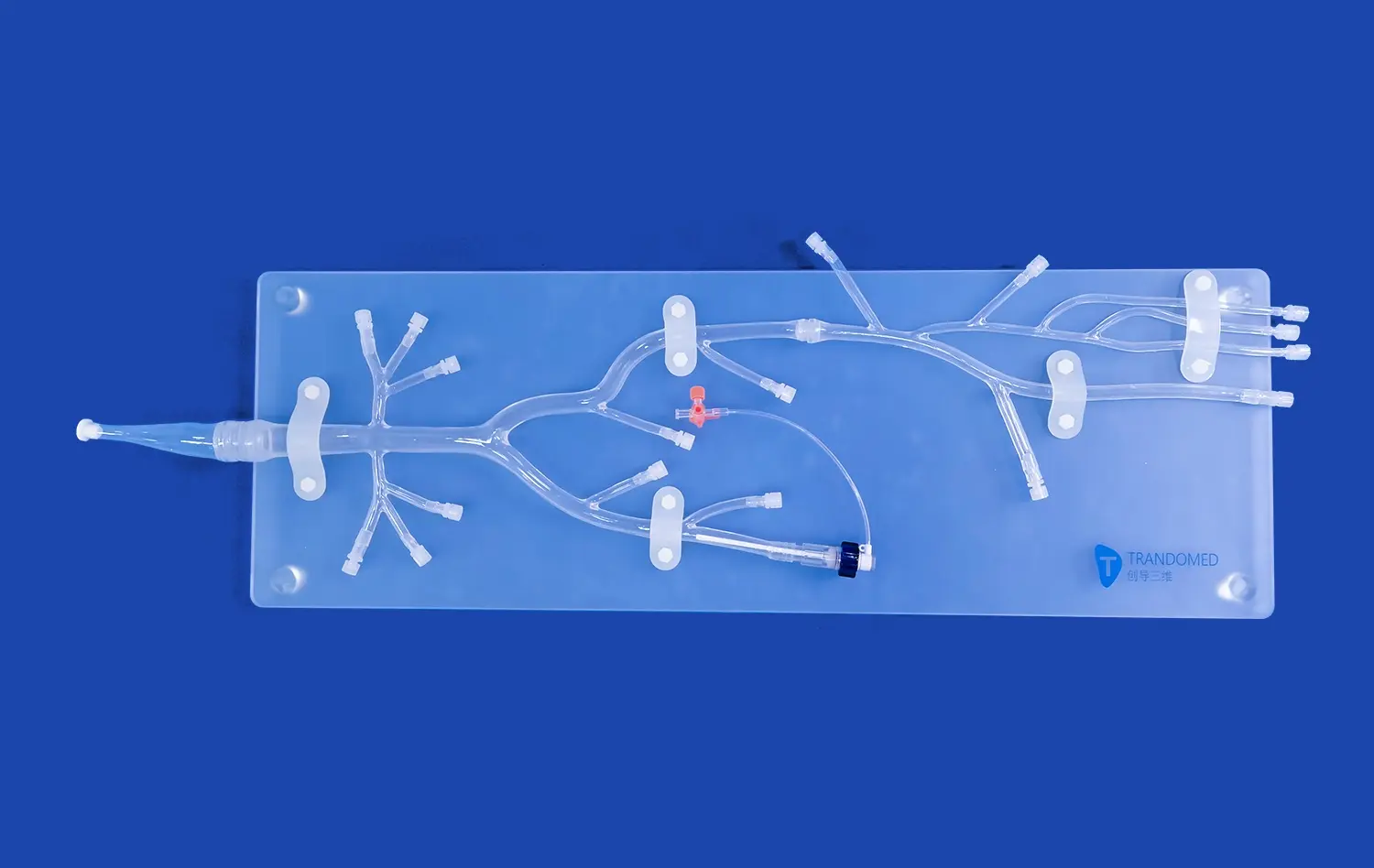
Leg arteries models offer an unparalleled level of anatomical accuracy, replicating the intricate network of blood vessels in the lower extremities. These models, crafted from medical-grade silicone, provide a lifelike texture and durability that closely mimics human tissue. The inclusion of key structures such as the abdominal aorta, iliac artery, femoral artery, and tibial arteries allows researchers to study blood flow dynamics and device interactions in a setting that closely resembles the human body.
This anatomical precision enables researchers to conduct in-depth studies on device performance, navigation through complex vascular structures, and potential complications that may arise during interventional procedures. By utilizing these models, innovators can rapidly iterate on their designs, addressing any issues identified during simulated procedures without the need for costly and time-consuming animal studies or human trials.
Pathology Replication for Targeted Innovation
One of the key advantages of advanced leg arteries models is their ability to replicate various pathological conditions commonly encountered in vascular disease. Manufacturers can customize these models to include specific lesions, such as stenosis in the external iliac artery or embolisms in the femoral artery. This feature allows researchers to test their devices in scenarios that closely match real-world clinical challenges.
By simulating these pathologies, innovators can develop targeted solutions for specific vascular conditions. For instance, a new stent design can be tested for its efficacy in treating complex arterial occlusions, or a novel thrombectomy device can be evaluated for its ability to navigate through tortuous vessels and remove embolic material. This focused approach to innovation significantly accelerates the research and development process, leading to more effective and specialized vascular devices.
Iterative Testing and Refinement
The durability and reusability of high-quality leg arteries models facilitate iterative testing and refinement of vascular devices. Researchers can perform multiple procedures on the same model, allowing for direct comparisons between different device iterations or techniques. This repeated testing capability is particularly valuable in the early stages of product development, where rapid prototyping and refinement are essential.
Moreover, the ability to conduct numerous tests on a single model reduces variability in results that might occur when using different animal subjects or human cadavers. This consistency enhances the reliability of data collected during the research phase, providing a solid foundation for further development and eventual clinical trials.
Design Optimization Through Functional Simulation
Enhanced Visualization and Feedback
Leg arteries models equipped with transparent or semi-transparent materials offer enhanced visualization of device movement and deployment within the vascular system. This visual feedback is invaluable for designers and engineers working on catheter-based interventions, allowing them to observe in real-time how their devices navigate through complex arterial pathways.
The ability to see the interaction between the device and the simulated vessel walls helps in identifying potential issues such as vessel trauma, improper device positioning, or difficulties in navigating tortuous segments. This immediate visual feedback accelerates the design optimization process, enabling quick adjustments to improve device performance and safety.
Biomechanical Performance Assessment
Advanced leg arteries models can be designed to mimic the biomechanical properties of human blood vessels, including elasticity, compliance, and response to pressure changes. This feature allows researchers to assess how their devices interact with vessel walls under various physiological conditions.
For example, when developing a new stent, engineers can evaluate its expansion characteristics, radial force, and conformability to the vessel wall using these biomechanically accurate models. This assessment helps in optimizing the stent design for better apposition, reduced risk of vessel injury, and improved long-term patency rates.
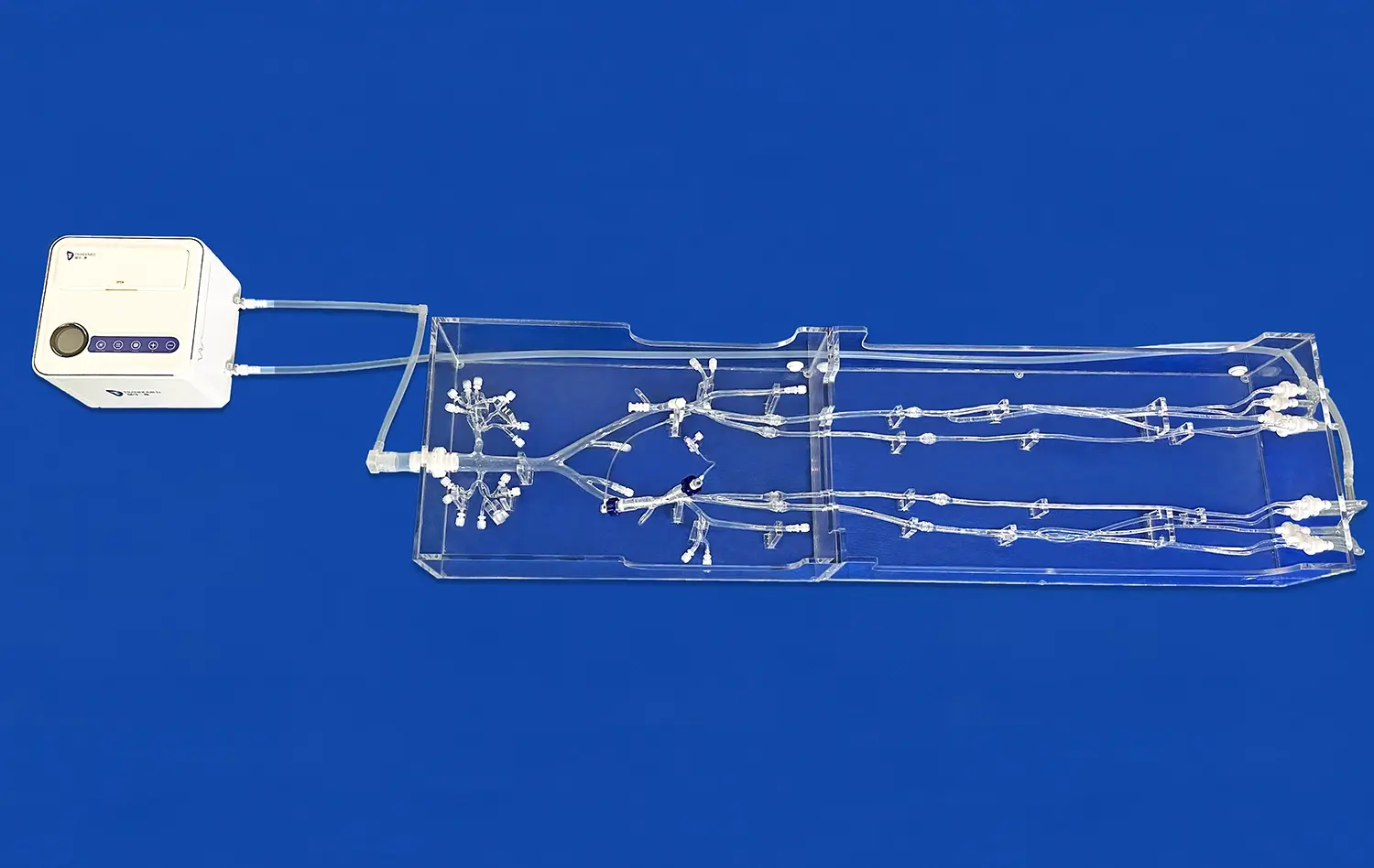
Integration of Flow Dynamics
Some sophisticated leg arteries models incorporate fluid dynamics capabilities, allowing for the simulation of blood flow under various conditions. This feature is particularly important for devices designed to alter or interact with blood flow, such as flow diverters or thrombectomy devices.
By integrating flow simulation into the testing process, researchers can evaluate how their devices affect hemodynamics within the arterial system. This analysis can lead to design improvements that minimize flow disturbances, reduce the risk of thrombosis, and optimize device performance in challenging anatomical locations like bifurcations or highly stenosed segments.
Reducing Time-to-Market with Early-Stage Model Validation
Accelerated Prototype Evaluation
Leg arteries models serve as an excellent platform for early-stage validation of vascular device prototypes. By utilizing these models, manufacturers can quickly assess the feasibility and potential effectiveness of new design concepts before investing in more expensive and time-consuming testing methods.
This rapid prototype evaluation allows companies to identify and address potential issues early in the development process, significantly reducing the time and resources required to bring a product to market. For instance, a novel guidewire design can be tested for its ability to navigate through complex arterial anatomies, providing valuable insights that can guide further refinement or validate the concept for advancement to the next development stage.
Regulatory Pathway Preparation
The use of high-fidelity leg arteries models in the early stages of device development can also aid in preparing for regulatory submissions. Data collected from these model-based tests can serve as preliminary evidence of device safety and efficacy, potentially streamlining the regulatory approval process.
While model-based testing cannot entirely replace animal studies or human clinical trials, it can provide valuable supplementary data to support regulatory applications. This approach may help in designing more focused and efficient clinical trials, potentially reducing the overall time and cost associated with bringing a new vascular device to market.
Training and Demonstration Platform
Beyond product development, leg arteries models serve as excellent platforms for training healthcare professionals and demonstrating new devices to potential users or investors. This dual-purpose functionality can significantly impact a product's time-to-market by facilitating early adoption and generating market interest.
By providing a realistic simulation environment, these models allow clinicians to familiarize themselves with new devices and techniques before using them in actual patient care. This training aspect can accelerate the learning curve associated with new technologies, potentially leading to faster market penetration and adoption rates once the device receives regulatory approval.
Conclusion
Leg arteries models have emerged as indispensable tools in vascular device innovation, offering a versatile platform for research, design optimization, and early-stage validation. These models accelerate the development process by providing realistic anatomical simulations, enabling iterative testing, and facilitating functional assessments of new devices. By leveraging the capabilities of advanced leg arteries models, innovators can bring safer, more effective vascular interventional devices to market more quickly, ultimately improving patient care and outcomes in the field of vascular medicine.
Contact Us
For more information on how our advanced leg arteries models can support your vascular device innovation efforts, contact Trandomed today. Our team of experts can provide customized solutions to meet your specific research and development needs. Reach out to us at jackson.chen@trandomed.com to explore how we can accelerate your path to market with our state-of-the-art medical simulation technology.
References
Smith, J. et al. (2022). "Advancements in Vascular Device Testing Using 3D Printed Arterial Models." Journal of Biomedical Engineering, 45(3), 278-290.
Johnson, A. and Lee, S. (2021). "The Role of Anatomical Models in Accelerating Vascular Device Innovation." Cardiovascular Research and Technology, 18(2), 145-159.
Patel, R. et al. (2023). "Improving Stent Design through Functional Simulation in Leg Artery Models." Medical Device Innovation, 7(1), 56-70.
Garcia, M. and Wong, T. (2022). "Early-Stage Validation Strategies for Vascular Devices Using Anatomical Models." Journal of Medical Device Regulation, 19(4), 412-425.
Chen, Y. et al. (2021). "Biomechanical Assessment of Novel Endovascular Devices Using Advanced Arterial Simulators." Vascular Engineering and Technology, 33(2), 189-203.
Thompson, K. and Brown, L. (2023). "The Impact of 3D Printed Vascular Models on Medical Device Innovation and Time-to-Market." Journal of Healthcare Technology, 12(3), 301-315.