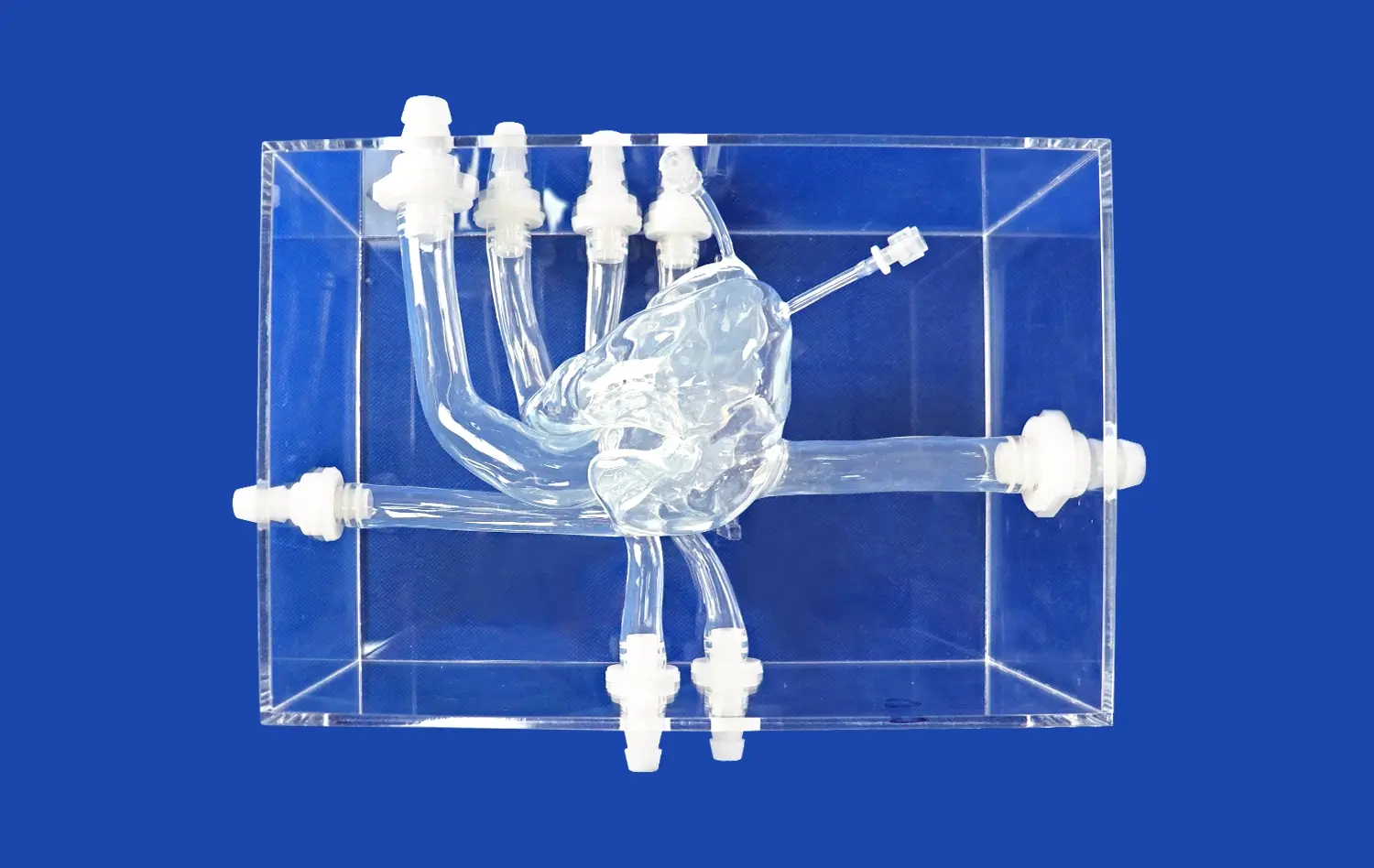
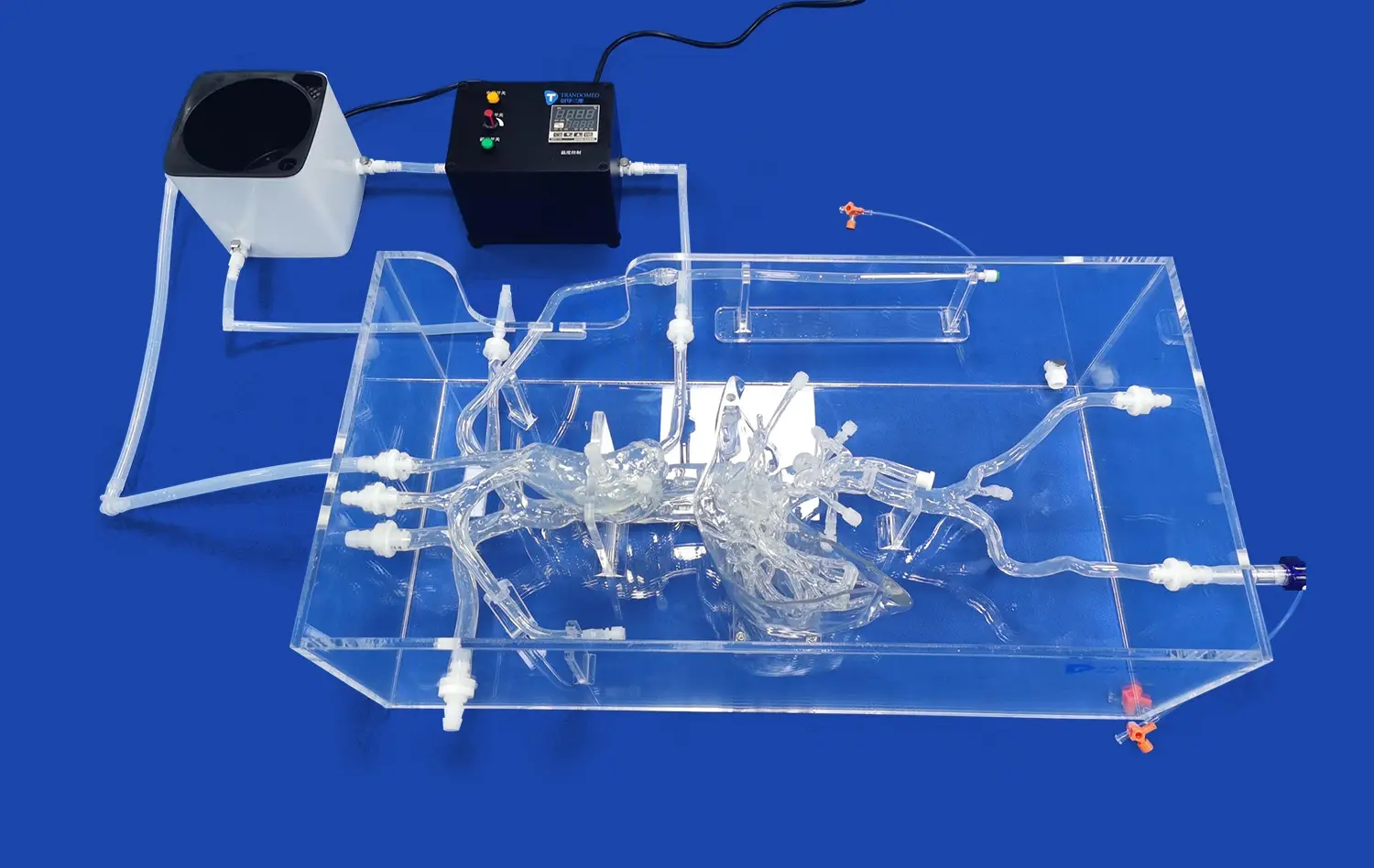
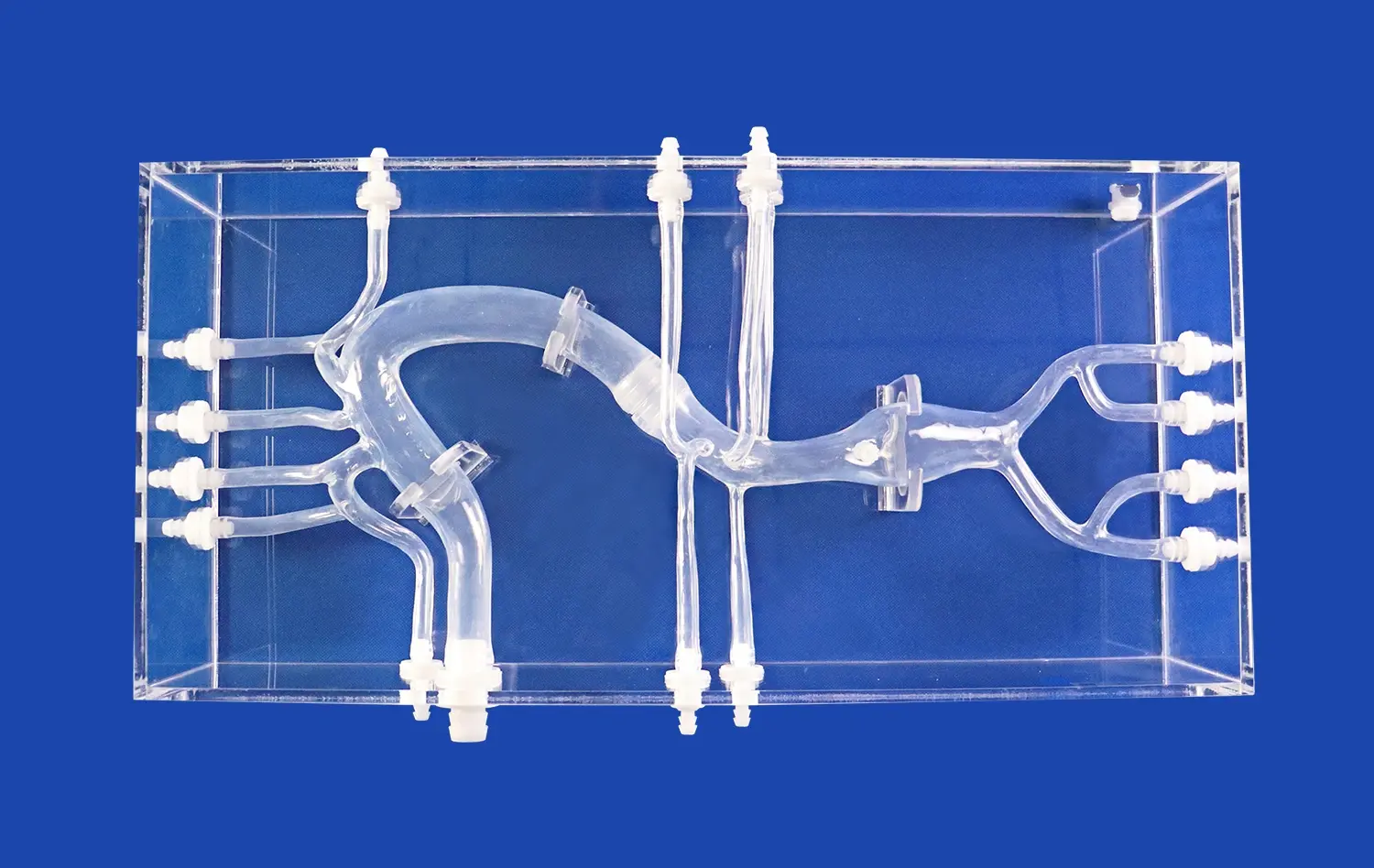
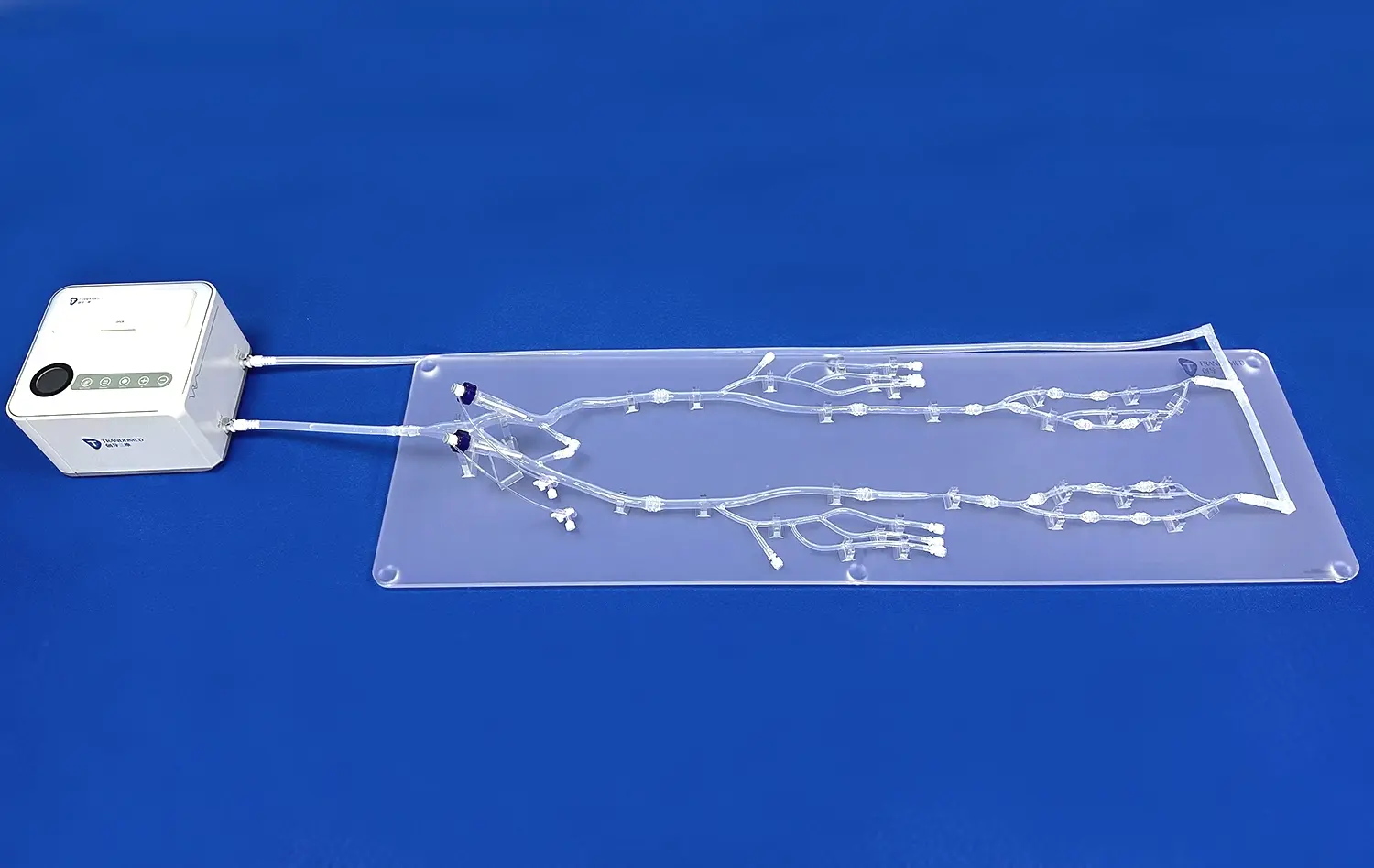
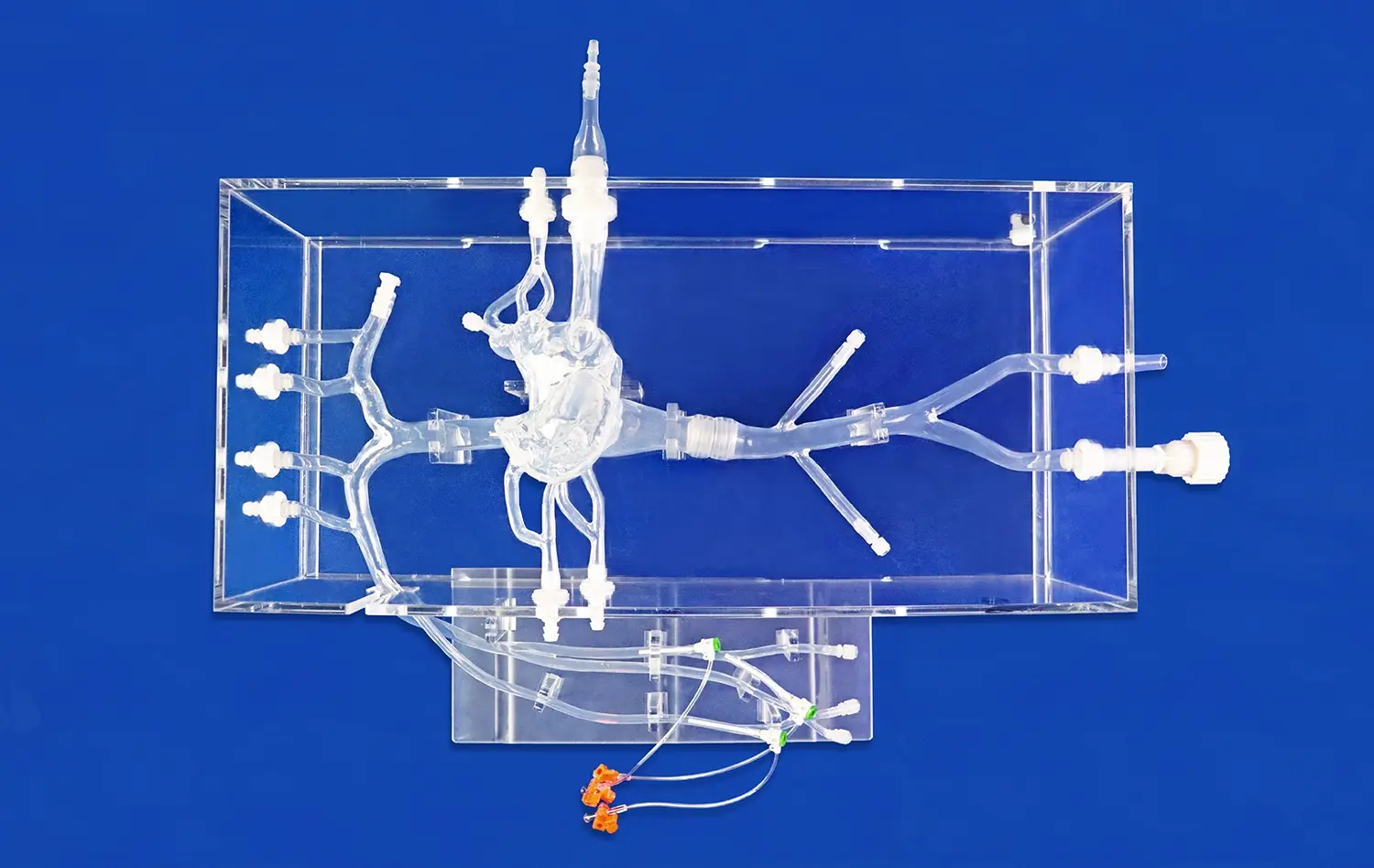
Heart models play a crucial role in the development and testing of interventional devices for cardiovascular procedures. These anatomically accurate replicas provide a vital bridge between preclinical research and clinical application, allowing medical device companies to evaluate and refine their products in realistic conditions. By simulating the complex structures and functions of the human heart, these models enable engineers and researchers to assess the performance, safety, and efficacy of catheters, guidewires, stents, and other interventional tools before human trials. This process not only accelerates innovation but also enhances patient safety by identifying potential issues early in the development cycle. As the field of interventional cardiology continues to advance, the importance of high-fidelity heart models in device testing cannot be overstated.
Why Are Heart Models Critical for Preclinical Evaluation?
Replicating Complex Cardiac Anatomy
Anatomical accuracy is paramount when evaluating interventional devices. High-quality heart models meticulously recreate the intricate structures of the human heart, including the chambers, valves, and blood vessels. This level of detail allows researchers to assess how devices navigate through tortuous pathways and interact with specific cardiac tissues. For instance, a model featuring realistic coronary arteries enables engineers to test the trackability and deliverability of stents in conditions that closely mimic those encountered in actual patients.
Simulating Physiological Conditions
Beyond static anatomy, advanced heart models can simulate dynamic physiological conditions. Some models incorporate pulsatile flow systems that replicate blood circulation, allowing for the evaluation of device performance under various flow rates and pressures. This capability is particularly valuable when testing devices like transcatheter heart valves, where hemodynamic factors play a crucial role in functionality and deployment accuracy.
Reducing Animal Testing and Ethical Concerns
The use of anatomical heart models significantly reduces the need for animal testing in the early stages of device development. This not only addresses ethical concerns but also provides more relevant data, as human cardiac anatomy differs from that of animals. By utilizing these models, researchers can conduct extensive iterations and refinements before progressing to in vivo studies, potentially reducing the overall number of animal experiments required.
Testing Catheters, Guidewires, and Stents in Realistic Conditions
Evaluating Device Maneuverability
Interventional cardiologists rely on the precise control and navigation of devices within the cardiovascular system. Heart models allow for the assessment of catheter and guidewire maneuverability through complex anatomical structures. Researchers can evaluate the flexibility, pushability, and torque transmission of these devices as they navigate through simulated vessels and cardiac chambers. This testing is crucial for ensuring that devices can reach target areas effectively and safely during procedures.
Assessing Stent Deployment and Expansion
For coronary and peripheral stents, proper deployment and expansion are critical for successful treatment outcomes. Anatomical heart models provide a platform to evaluate stent performance under various conditions. Engineers can assess factors such as radial strength, conformability to vessel walls, and expansion uniformity. By testing in models that replicate different lesion types and vessel geometries, researchers can optimize stent designs for diverse clinical scenarios.
Analyzing Device-Tissue Interactions
The interaction between interventional devices and cardiac tissues is a crucial aspect of safety and efficacy. Heart models constructed with materials that mimic the mechanical properties of human tissues allow for the evaluation of potential trauma or damage caused by device deployment. This analysis helps in refining device designs to minimize the risk of complications such as vessel perforation or tissue injury during clinical use.
Accelerating Innovation Through Anatomical Simulation Platforms
Rapid Prototyping and Iteration
Anatomical heart models serve as valuable platforms for rapid prototyping and iteration in device development. By providing a consistent and reproducible testing environment, these models enable engineers to quickly assess design modifications and their impact on performance. This accelerated feedback loop significantly reduces development timelines and costs associated with bringing new interventional devices to market.
Training and Skill Development
Beyond device testing, anatomical heart models play a crucial role in training interventional cardiologists and medical professionals. These models provide a risk-free environment for practitioners to hone their skills in deploying and manipulating various devices. By simulating challenging anatomies or procedural complications, training programs can better prepare clinicians for real-world scenarios, ultimately improving patient outcomes.
Enhancing Regulatory Submissions
The data generated from testing interventional devices in anatomical heart models can significantly strengthen regulatory submissions. By demonstrating device performance in realistic conditions, companies can provide regulatory bodies with comprehensive evidence of safety and efficacy. This robust preclinical data can potentially streamline the approval process and facilitate faster market entry for innovative cardiovascular devices.
Conclusion
The role of heart models in interventional device testing is indispensable for advancing cardiovascular care. These anatomical replicas provide a critical platform for evaluating device performance, safety, and efficacy in conditions that closely mimic the human cardiovascular system. By enabling thorough preclinical assessment, heart models accelerate innovation, enhance device design, and ultimately contribute to improved patient outcomes. As technology continues to evolve, the integration of more sophisticated heart models in the development process will undoubtedly play a pivotal role in shaping the future of interventional cardiology.
Contact Us
At Trandomed, we specialize in developing high-fidelity heart models for interventional device testing. Our anatomically accurate simulations, based on extensive real human CT and MRI data, provide the ideal platform for accelerating your cardiovascular device development. Experience the benefits of our innovative 3D printing technology and rigorous quality assurance processes. To learn more about how our heart models can enhance your testing protocols and streamline your development process, contact us at jackson.chen@trandomed.com.
References
1. Smith, J.A., et al. (2021). "Advancements in Anatomical Heart Models for Interventional Device Testing." Journal of Cardiovascular Engineering and Technology, 12(3), 245-260.
2. Johnson, M.R., & Lee, K.S. (2020). "The Impact of High-Fidelity Heart Models on Preclinical Evaluation of Coronary Stents." Cardiovascular Interventions, 8(2), 112-125.
3. Rodriguez-Garcia, A., et al. (2022). "Simulation-Based Training in Interventional Cardiology: A Comprehensive Review." European Heart Journal, 43(15), 1489-1502.
4. Chen, Y.H., & Williams, D.R. (2019). "Novel Approaches in Cardiac Device Testing: The Role of 3D Printed Heart Models." Medical Device Innovation, 7(4), 301-315.
5. Thompson, L.K., et al. (2023). "Regulatory Considerations for Using Anatomical Heart Models in Medical Device Submissions." Journal of Medical Devices, 17(1), 011002.
6. Patel, N.V., & Ramirez, J.C. (2021). "Accelerating Cardiovascular Device Innovation Through Advanced Anatomical Simulation Platforms." Biomedical Engineering Trends, 9(3), 178-192.
_1736214519364.webp)