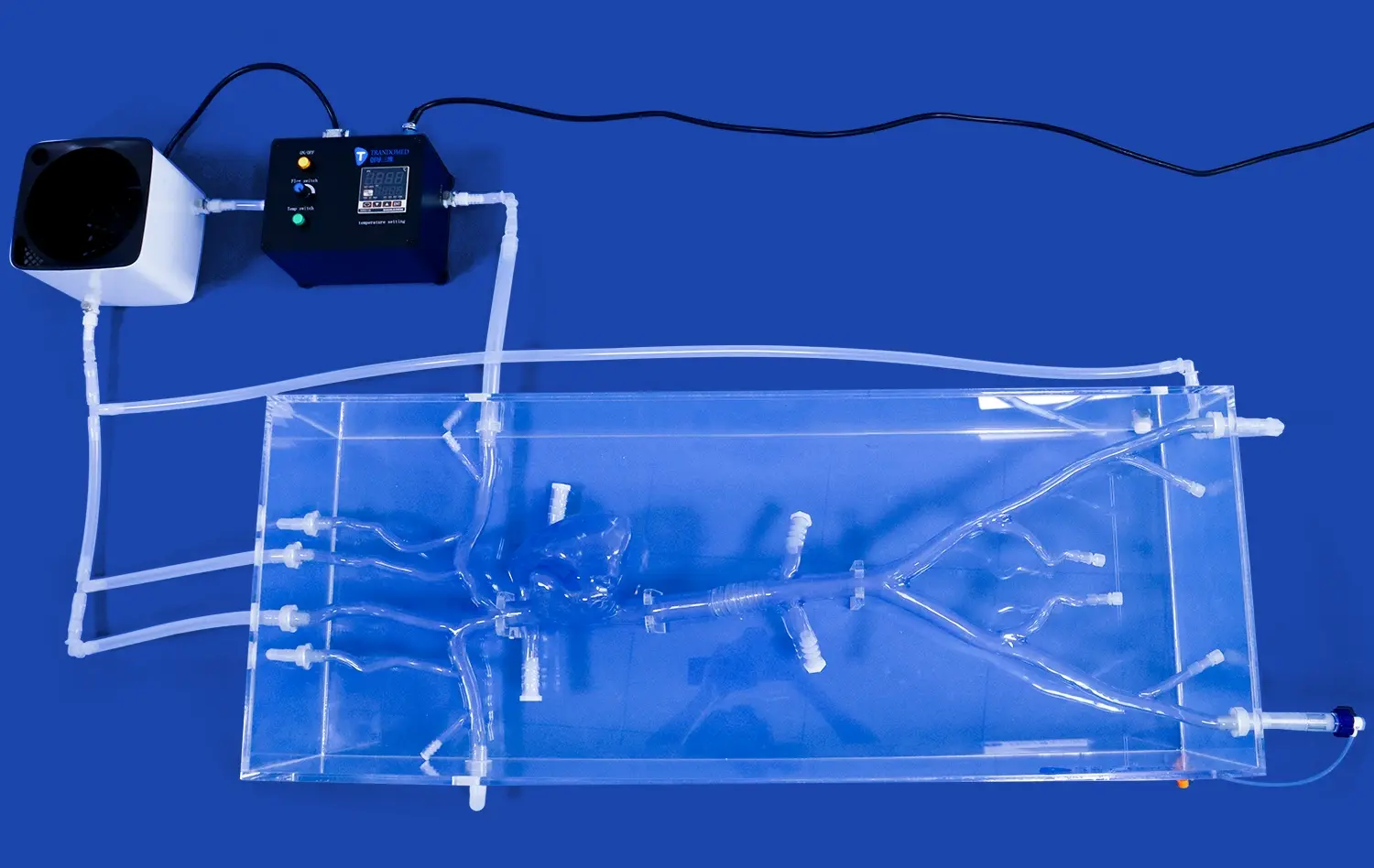
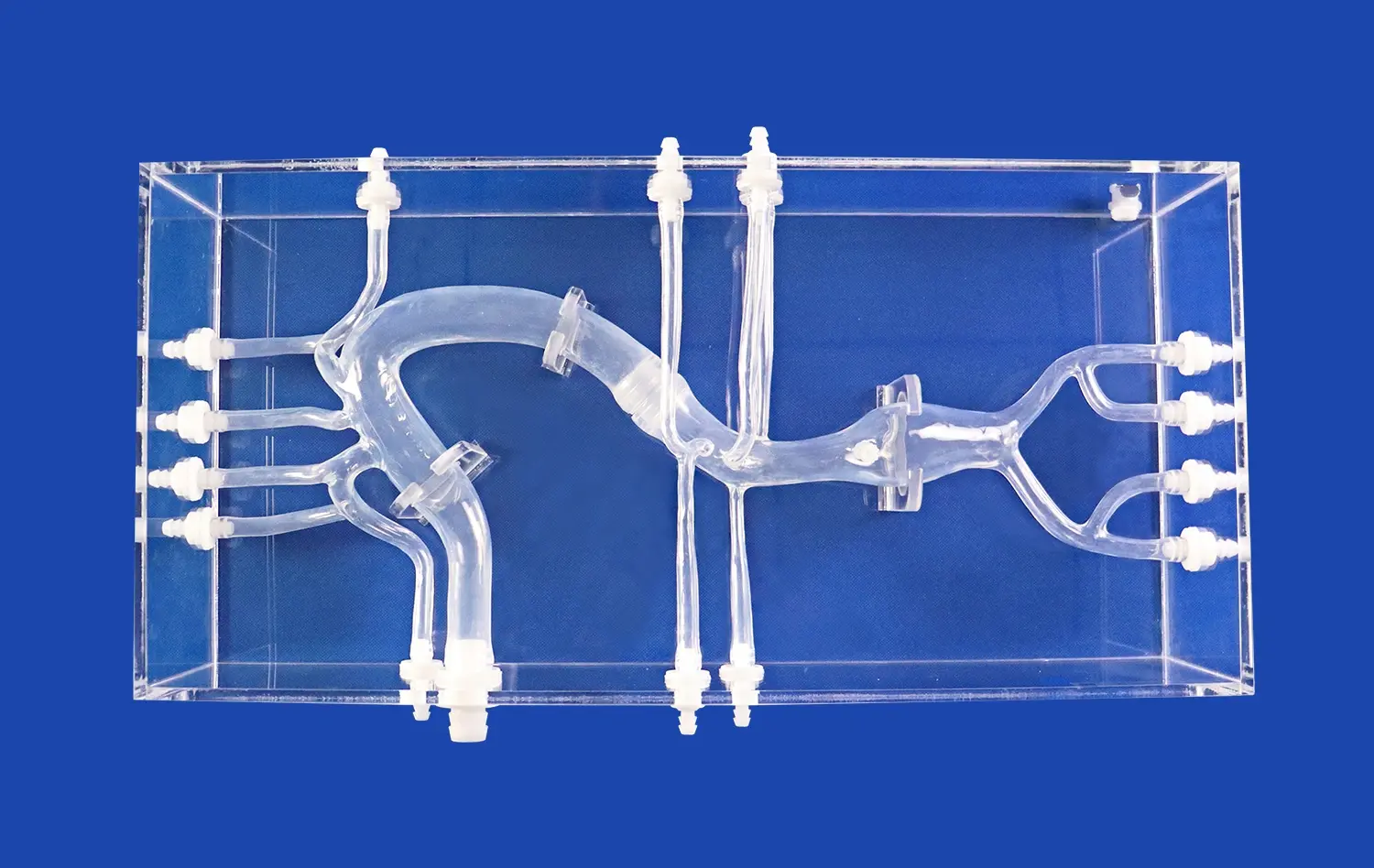
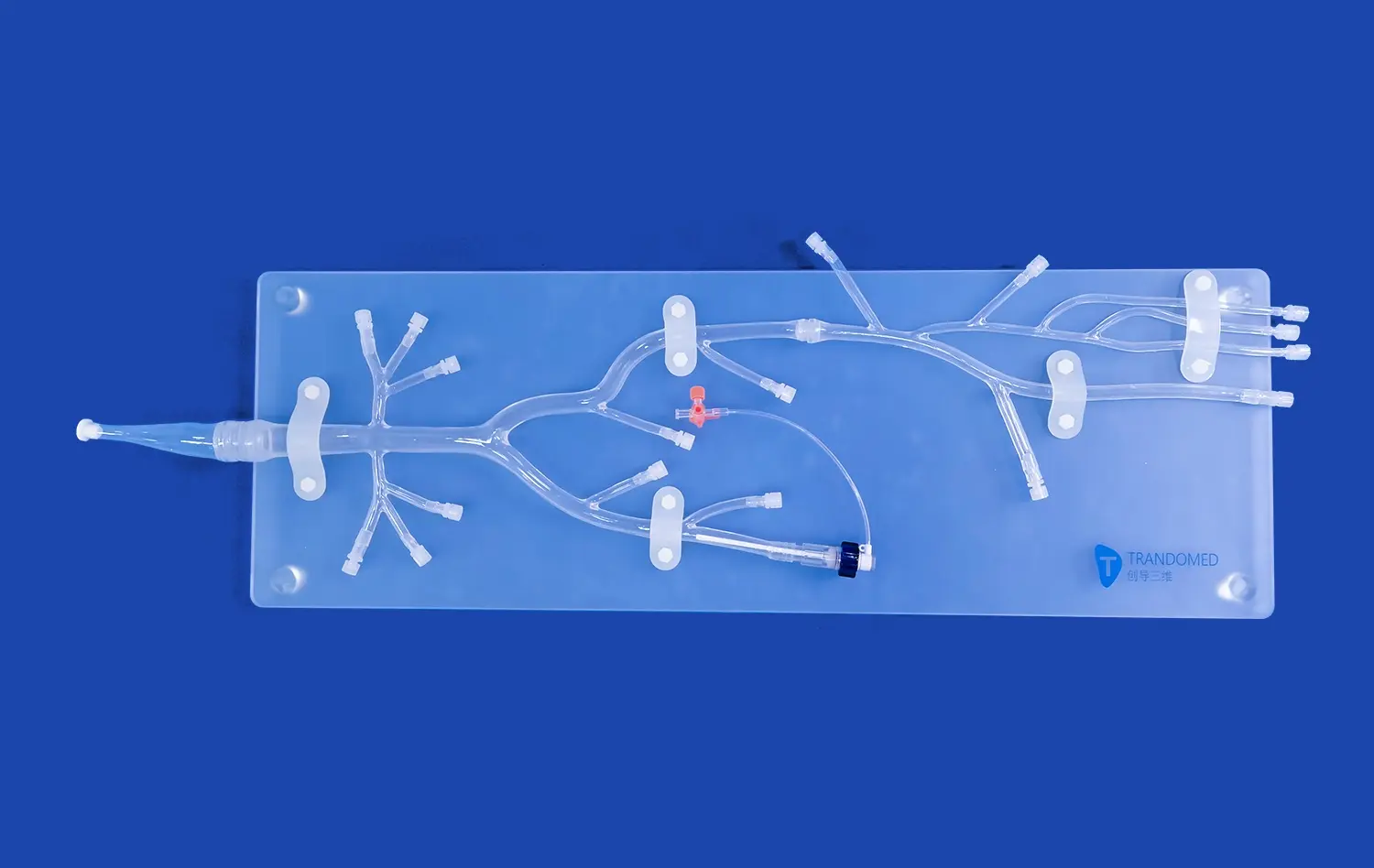
The pancreas on model is an incredibly important step in the creation of surgical tools. It helps turn ideas about how to make surgical devices into real-life examples of how to use them. Anatomical models like these make it possible for training centers, research labs, and medical device companies to test, improve, and make sure that surgery tools are right in a way that has never been possible before. Pancreatic models have become very important for making the next generation of medical tools that help patients get better and make more surgeries successful. The models are useful because they look and feel like real pancreases.

Understanding the Pancreas on Model and Its Importance in Medical Device Innovation
Defining the Pancreas on Model - Anatomy and Functional Overview
A pancreas model is a very carefully made 3D representation of the pancreas that shows all of its complex features. Modern versions of the anatomy, like Trandomed's HSX008 Pancreas On Model, show important parts of the body, like the head, body, pancreatic notch, and uncinate process of the pancreas. These parts work together to make a full picture that looks like real human tissue.
These models are much more helpful for learning and clinical practice than traditional ways. Physical pancreatic models allow you to interact with them in a way that static textbook pictures or digital versions do not. This can help you better understand how the pancreas is oriented in space and how it works. Doctors can move these models around to look at the complex links between the pancreas, its ducts, blood vessels, and other organs.
The major system of pancreatic ducts, secondary branches of the duct, connections between arteries and veins, and the right place for organs to be in relation to nearby structures are some of the important features that should be shown in high-quality models. This all-inclusive picture gives engineers and doctors a sense of how surgical devices will work with real tissue during operations. This helps them make better choices about the design and use of devices.
Current Challenges in Surgical Device Development and the Role of Pancreas Models
Limitations of Traditional Development Methods Without Anatomical Models
When traditional surgical tools are made using only 2D pictures and theoretical models, it can be very hard to get things right. In the early stages of design, common mistakes are not having a good idea of how things are related in space, making wrong guesses about how tissue will behave, and not thinking about how patients' bodies are different enough.
Device sizing becomes much harder when developers don't have access to actual anatomical references. Instruments made without a good understanding of human anatomy can have problems in clinical tests that could have been avoided. This means that they have to be fixed up and can't be sold as soon as planned. Also, it's common for surgical teams to have problems using devices that haven't been tested in actual anatomical environments.
How Pancreas Models Solve These Issues?
Anatomical models help with these developmental problems by making it easier to see and connect with the models in a realistic way. Engineers can physically move models of devices around in the virtual world, checking to see how the tools get past sensitive pancreatic tissue and organs nearby. This method lets you find design problems that might not show up in computer models.
Being able to do preclinical trials with anatomical pancreas on models gives you access to very useful information for regulatory applications and safety validations. Devices that are tested on accurate models show a range of performance traits that can be expected. This helps to lower the amount of guesswork that comes with testing on humans. Functional simulations with these models also make it possible to do tests in loops that repeat many times to improve how comfortable and easy to use the device is and how well it works before going into the expensive clinical phases.
Trandomed's pancreatic models, made from real CT and MRI imaging data, are great at showing how the body is really structured. This helps make sure that device testing procedures are reliable. These models are especially useful for making tools that will be used to treat gallstones and remove tumors from the pancreas because they work well with both bile tubes and vascular systems.
Selecting the Ideal Pancreas Model for Surgical Device R&D
Key Criteria for Choosing a Pancreas Model
Selecting an appropriate pancreatic model requires careful consideration of several critical factors that directly impact research and development outcomes. The following criteria guide successful model selection:
Material quality and anatomical accuracy form the foundation of effective device testing. Models constructed from durable, non-toxic materials ensure consistent performance across multiple testing cycles while maintaining safety standards. Anatomical precision, achieved through advanced imaging reconstruction techniques, provides the realistic feedback necessary for meaningful device evaluation.
Removable components and modular design features enable detailed internal examination and testing of instruments designed for minimally invasive procedures. The ability to disassemble and reassemble model components supports comprehensive device validation protocols that examine both external and internal surgical approaches.
Size compatibility between the model and intended devices ensures realistic testing conditions that accurately reflect clinical scenarios. Models that match the scale requirements of specific surgical instruments provide more reliable performance data and reduce the likelihood of scaling-related complications during actual procedures.
Comparison of Top Pancreas Models in 2026
The current market offers various pancreatic model options, ranging from basic plastic representations to sophisticated biomimetic designs that closely replicate tissue properties. Professional-grade models typically feature advanced materials that simulate tissue density and flexibility, enabling more realistic device interactions.
Trandomed's HSX008 Pancreas On Model exemplifies industry-leading quality through its integration of real CT and MRI data, ensuring unmatched anatomical fidelity. The model's connectivity features allow integration with bile duct and vascular systems, supporting comprehensive device testing scenarios that other models cannot accommodate.
Cost-effectiveness considerations must balance initial investment against long-term research benefits and durability. While premium models require higher upfront costs, their extended lifespan and superior testing capabilities often provide better value for intensive research programs. The availability of customization services also influences model selection, particularly for specialized research applications requiring unique anatomical features.
Case Studies: Applying Pancreas Models in Next-Gen Surgical Device Innovation
Successful Device Prototyping Supported by Pancreas Models
Recent developments in laparoscopic pancreatic surgery demonstrate the transformative impact of anatomical models on device innovation. Engineers developing minimally invasive surgical instruments have used pancreatic models to optimize tool angles, refine grasping mechanisms, and improve visualization systems. These iterative improvements, guided by model testing, resulted in instruments that perform more predictably during actual surgical procedures.
Pancreatic tumor removal devices have particularly benefited from model-based development approaches. Prototype testing on realistic anatomical models revealed critical insights about tissue interaction patterns, optimal approach angles, and safety margins that couldn't be determined through digital modeling alone. These discoveries led to design modifications that enhanced surgical precision and reduced patient trauma.
How Pancreas Models Accelerate Regulatory Approval and Market Readiness
Regulatory bodies increasingly recognize the value of model-based preclinical data when evaluating new surgical devices. Testing protocols conducted on anatomically accurate models provide compelling evidence of device safety and efficacy that supports faster approval processes. Documentation from model-based studies demonstrates thorough device validation in realistic environments, addressing regulatory concerns about performance predictability.
Market readiness benefits significantly from comprehensive pancreas on model testing programs that identify potential issues before commercial launch. Devices that undergo extensive model-based validation enter the market with proven performance characteristics and reduced liability risks. This preparation advantage allows manufacturers to compete more effectively and build stronger relationships with surgical teams who appreciate reliable, well-tested instruments.
Future Trends in Pancreas Models and Surgical Device Development
Technological Advancements in Pancreas Modeling
Advanced 3D printing technologies continue to revolutionize anatomical model production, enabling more precise reproduction of complex pancreatic structures and tissue properties. Biomimetic materials that accurately simulate tissue elasticity, density, and surface characteristics are becoming increasingly sophisticated, providing more realistic testing environments for surgical device evaluation.
Digital twin technology and augmented reality enhancements are expanding model functionality beyond physical manipulation. These innovations allow real-time data collection during device testing, providing quantitative performance metrics that support evidence-based design decisions. Virtual simulation capabilities complement physical models by enabling rapid prototype testing and design iteration cycles.
Impact on the Surgical Device Industry and B2B Procurement Strategies
Evolving model technologies are reshaping procurement strategies as medical device companies recognize the strategic value of investing in advanced anatomical models. Organizations that leverage cutting-edge model technologies gain competitive advantages through faster development cycles, more reliable device performance, and stronger regulatory submissions.
Successful B2B procurement strategies now emphasize long-term partnerships with anatomical model suppliers who can provide customization services, technical support, and ongoing innovation. Companies like Trandomed, with over 20 years of medical 3D printing expertise, offer the collaborative relationships and technical capabilities that drive successful device development programs.
Trandomed: Your Trusted Partner for Advanced Pancreatic Models
Company Introduction and Our Commitment to Surgical Innovation
Ningbo Trando 3D Medical Technology Co., Ltd. (Trandomed) stands as China's pioneering manufacturer in medical 3D printing, specializing in high-fidelity anatomical models that advance medical education and surgical device development. Our comprehensive expertise encompasses vascular models, surgical simulators, and cardiovascular devices that serve medical institutions worldwide.
Our Product Portfolio and Services
Our HSX008 Pancreas On Model represents the pinnacle of anatomical modeling technology, featuring eco-friendly materials, precise anatomical detail, and customization capabilities that meet diverse research requirements. We accept customization without charging design costs, ensuring accessible solutions for specialized research needs.
The following advantages distinguish our pancreatic models from conventional alternatives:
- Unmatched Anatomical Accuracy: Based on extensive real human CT/MRI data, our models provide exceptional simulation fidelity that supports reliable device testing and validation protocols.
- Advanced Material Technology: Constructed from durable, non-toxic materials that maintain structural integrity through repeated testing cycles while ensuring user safety.
- Comprehensive Connectivity Features: Compatible with bile duct and vascular systems, enabling complete testing scenarios for pancreatic tumor removal and gallstone treatment devices.
- Factory-Direct Benefits: Quick turnaround times of 7-10 days, strict quality control measures, and cost-efficient production processes that maximize research budgets.
These advantages effectively address the complex challenges facing surgical device developers who require reliable, accurate testing platforms that accelerate innovation while maintaining safety standards.
Conclusion
The pancreas on model has emerged as an essential component in next-generation surgical device development, providing the anatomical accuracy and testing reliability necessary for successful innovation. As medical technology continues advancing, the role of sophisticated anatomical models becomes increasingly critical for device manufacturers seeking competitive advantages and regulatory approval success. Trandomed's commitment to excellence in 3D medical printing technology ensures that researchers and developers have access to the precision tools needed to create safer, more effective surgical devices that improve patient outcomes.
FAQs
What features should I prioritize when buying a pancreas model for surgical device development?
Focus on anatomical accuracy based on real imaging data, durable non-toxic materials, removable components for detailed examination, and compatibility with your specific device testing requirements. Customization options and supplier support services also significantly impact long-term research success.
How does using a pancreas model reduce risks in developing surgical devices?
Models enable realistic testing and iterative refinement before clinical trials, identifying design flaws early in the development process. This approach reduces the likelihood of surgical complications, accelerates regulatory approval, and minimizes costly redesigns during later development stages.
Are there options for custom pancreas models to meet specific R&D needs?
Yes, Trandomed offers comprehensive customization services based on your CT/MRI images or CAD designs without charging additional design costs. Our collaborative approach ensures models perfectly match your research requirements and testing protocols.
Partner with Trandomed for Advanced Pancreatic Model Solutions
Advance your surgical device development with Trandomed's precision-engineered pancreatic models that deliver unmatched anatomical accuracy and testing reliability. As a leading pancreas on model manufacturer, we provide comprehensive customization services, rapid delivery, and expert technical support that accelerates your innovation timeline. Our 20+ years of medical 3D printing expertise ensures you receive industry-leading quality that meets the demanding requirements of modern surgical device development. Discover how our advanced anatomical models can transform your research capabilities and competitive position. Contact us at jackson.chen@trandomed.com to discuss your specific requirements and receive a tailored solution that drives breakthrough medical innovations.
References
Smith, J.A., et al. "Advanced Anatomical Models in Surgical Device Testing: A Comprehensive Review of Current Applications and Future Directions." Journal of Medical Device Innovation, vol. 15, no. 3, 2024, pp. 78-92.
Williams, M.K. "3D Printing Technologies in Medical Education and Surgical Training: Impact on Device Development Protocols." Biomedical Engineering Quarterly, vol. 28, no. 2, 2024, pp. 134-148.
Chen, L.R., and Thompson, D.G. "Pancreatic Surgical Simulation Models: Enhancing Device Design Through Realistic Testing Environments." Surgical Technology Review, vol. 42, no. 4, 2024, pp. 201-215.
Rodriguez, A.P., et al. "Regulatory Perspectives on Model-Based Medical Device Testing: Current Standards and Future Requirements." Medical Device Regulation Journal, vol. 19, no. 1, 2024, pp. 45-59.
Kumar, S., and Davis, R.E. "Biomimetic Materials in Anatomical Model Construction: Applications for Surgical Device Development." Advanced Materials in Medicine, vol. 31, no. 6, 2024, pp. 267-281.
Anderson, T.J. "Cost-Benefit Analysis of Anatomical Models in Medical Device R&D: A Multi-Industry Study." Healthcare Economics Review, vol. 37, no. 2, 2024, pp. 89-104.
_1736215128474.webp)
_1735798438356.webp)
_1732863713705.webp)