The Role of PCI Training Models in Advancing Interventional Cardiology Devices
2025-08-18 09:00:02
PCI training models play a crucial role in advancing interventional cardiology devices by providing a realistic and risk-free environment for testing, refining, and validating new technologies. These sophisticated simulators, meticulously designed to replicate human coronary anatomy, serve as invaluable tools for device manufacturers, researchers, and clinicians alike. By enabling early-stage testing, accelerating design feedback loops, and supporting preclinical validation, PCI models significantly contribute to the development of innovative cardiac interventional devices. This article explores how these advanced training systems are shaping the future of interventional cardiology and improving patient outcomes.
How Do PCI Models Facilitate Early-Stage Device Testing?
Anatomical Accuracy for Realistic Evaluation
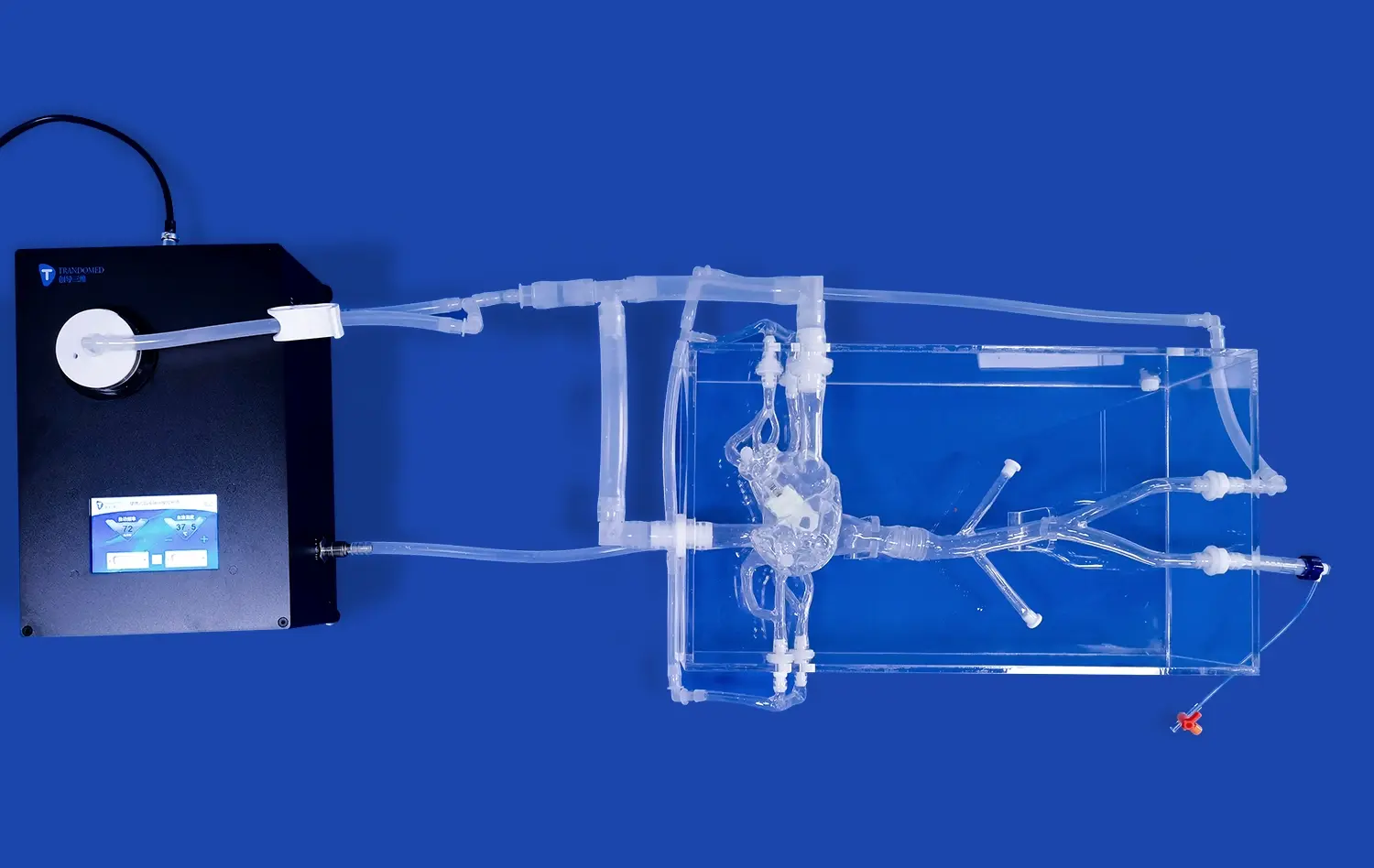
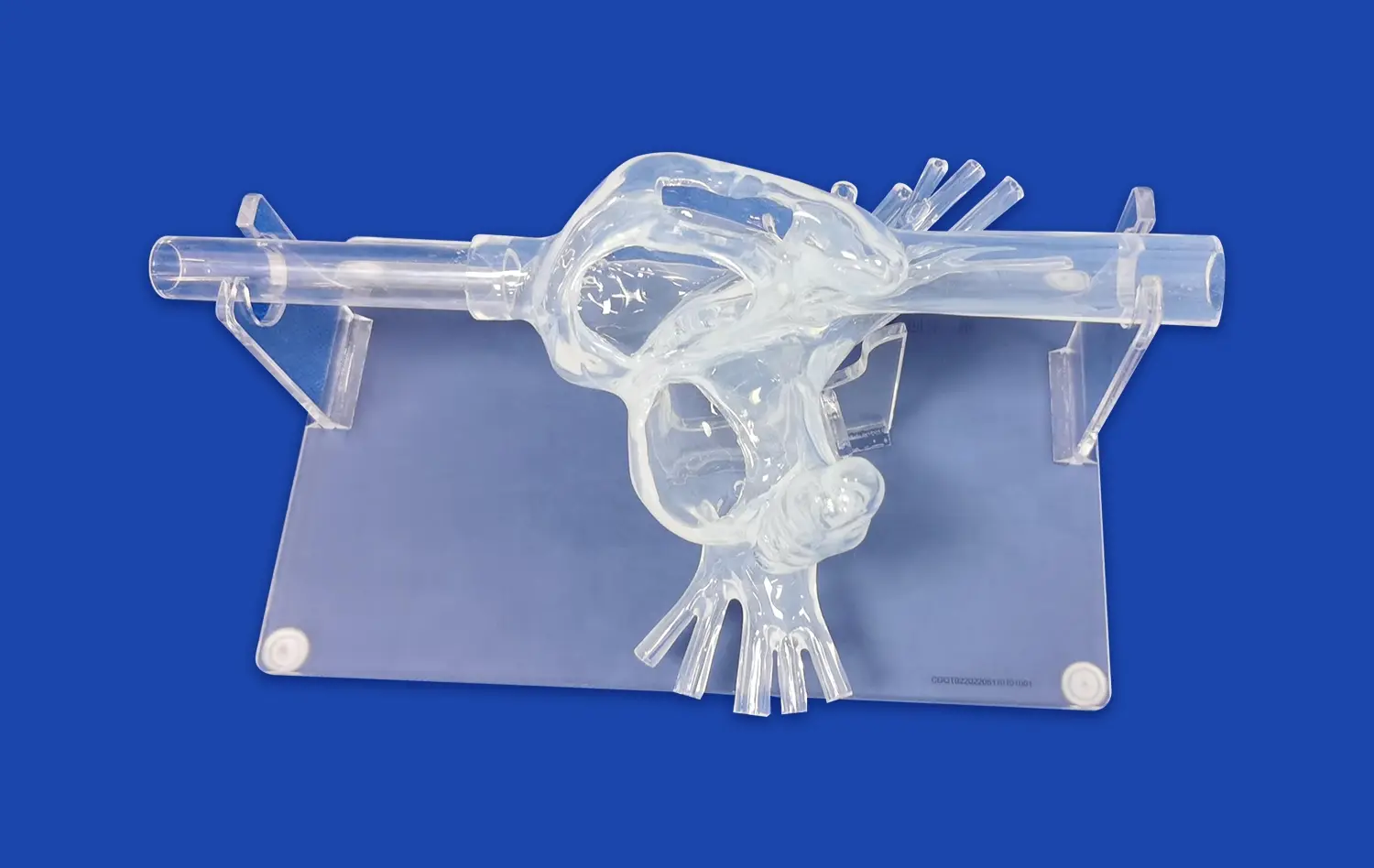
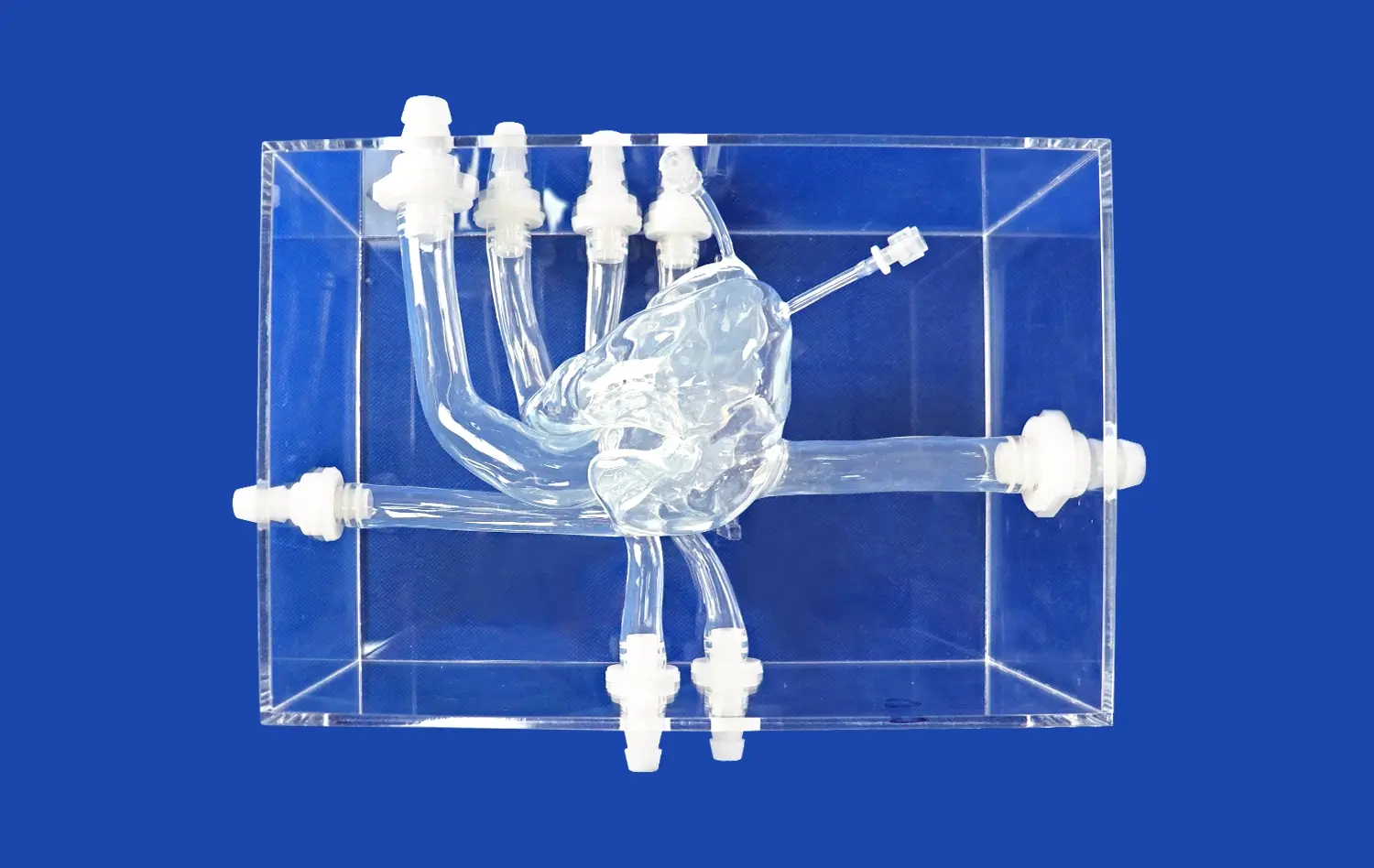
PCI training models provide exceptional anatomical fidelity, meticulously replicating the complex structure of human coronary arteries. Constructed using high-resolution CT and MRI data, these models offer true-to-life representations of critical features like vessel tortuosity, precise bifurcation angles, physiological tapering, and varying degrees of stenosis severity. This unprecedented level of detail allows device manufacturers to rigorously evaluate prototype performance – including trackability, deliverability, deployment mechanics, and interaction with simulated vessel walls – within an environment that closely mirrors actual patient anatomy. Such realistic simulation yields invaluable predictive insights into device behavior during clinical use, far surpassing simpler benchtop testing.
Customizable Pathologies for Comprehensive Testing
A defining strength of advanced PCI simulators' PCI training model is their capacity to be precisely customized with a wide array of clinically relevant coronary pathologies. Manufacturers can programmatically introduce specific lesion characteristics, such as heavily calcified plaques, dense fibrotic tissue, challenging chronic total occlusions (CTOs), complex bifurcations requiring specialized techniques, or diffuse disease. This targeted pathology replication enables exhaustive, scenario-specific testing across the full spectrum of clinical challenges. It ensures that new interventional devices are thoroughly vetted for efficacy, safety, and versatility against the diverse lesion types they will encounter in real-world patient populations before clinical trials commence.
Repeatability and Standardization in Testing Protocols
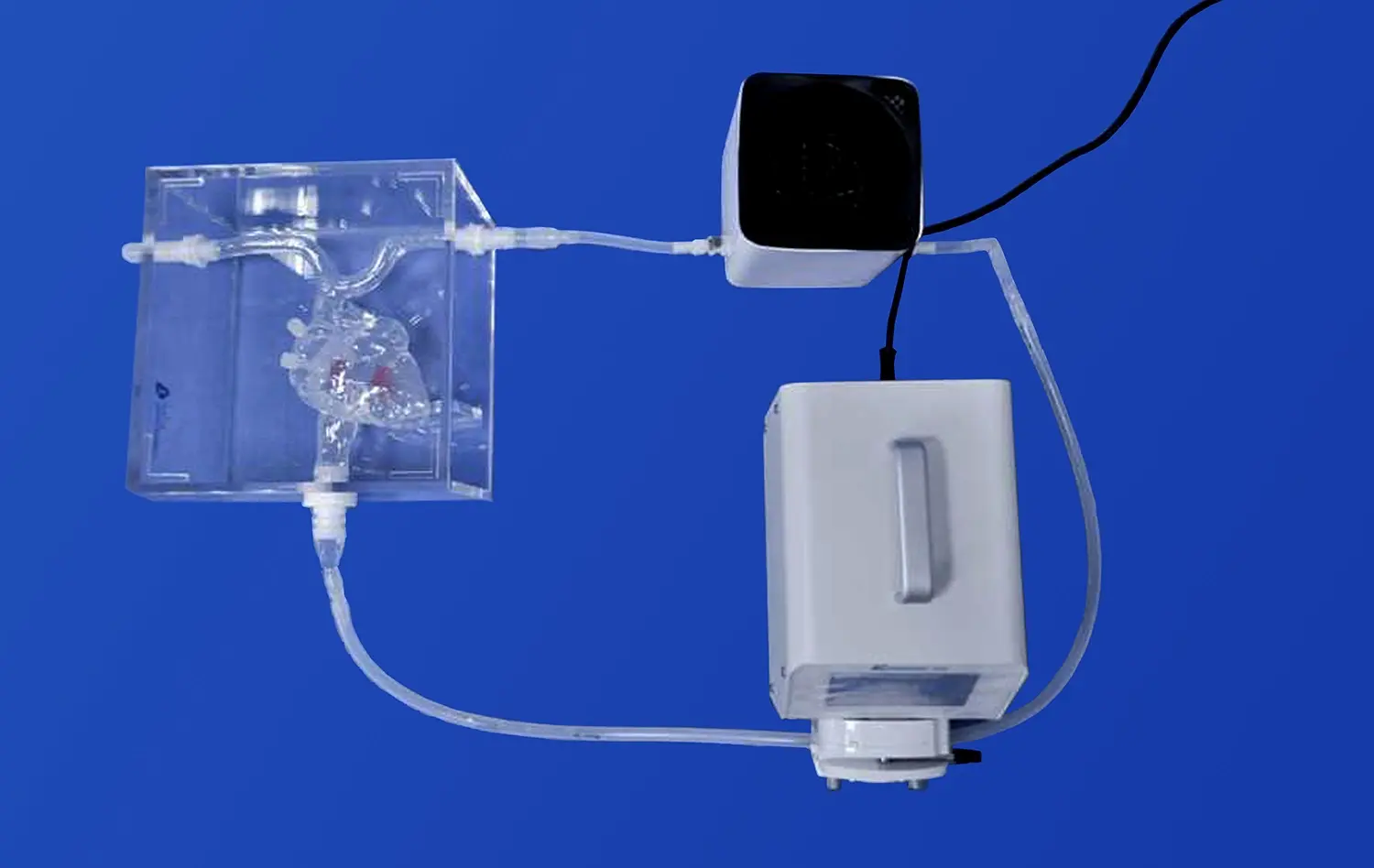
PCI models establish a crucial standardized and repeatable testing platform, eliminating the inherent variability of human clinical trials. This consistency is fundamental for reliable comparative analysis, whether evaluating sequential iterations of a single device during development or benchmarking against competitor products. By removing patient-specific variables (e.g., anatomy, physiology, co-morbidities), these simulators enable highly objective assessments under strictly controlled conditions. This rigorous approach facilitates the detection of even incremental improvements in performance metrics like deliverability scores, radial strength, conformability, or safety profiles, driving evidence-based design refinement and objective performance claims.
Accelerating Design Feedback Loops for Innovation
Rapid Prototyping and Iteration
The use of PCI training models significantly accelerates the design feedback loop in device development. Manufacturers can quickly test prototypes, gather data on performance, and make iterative improvements without the lengthy timelines associated with clinical trials. This rapid prototyping process allows for more agile development, enabling innovators to refine their designs based on immediate feedback from simulated interventions.
Multi-disciplinary Collaboration
PCI simulators serve as a common ground for collaboration between engineers, clinicians, and researchers. These models, such as PCI training models, allow for hands-on demonstrations and discussions, fostering a shared understanding of device functionality and clinical applications. This multi-disciplinary approach often leads to novel insights and innovative solutions that might not emerge in siloed development processes.
Cost-effective Innovation
By leveraging PCI training models, device manufacturers can significantly reduce the costs associated with early-stage development. These simulators minimize the need for animal studies and reduce the risk of costly design flaws being discovered late in the development process. This cost-effectiveness allows companies to explore more innovative ideas and push the boundaries of interventional cardiology technology.
Supporting Regulatory Approval Through Preclinical Validation
Robust Safety Assessment
PCI models play a crucial role in the preclinical validation of new interventional devices, providing a platform for comprehensive safety assessments. Manufacturers can use these simulators to evaluate potential risks, such as vessel perforation, dissection, or embolization, under various conditions. This thorough safety testing is essential for building a strong case for regulatory approval and ensuring patient safety.
Efficacy Demonstration in Simulated Clinical Scenarios
Regulatory bodies often require evidence of device efficacy before granting approval. PCI training models allow manufacturers to demonstrate the effectiveness of their devices across a range of simulated clinical scenarios. By documenting successful interventions in challenging anatomies or complex lesions, companies can provide compelling evidence of their device's potential clinical impact.
Training and Proficiency Validation
Beyond device testing, PCI simulators are instrumental in developing and validating training protocols for new interventional technologies. These models enable manufacturers to create standardized training programs, ensuring that clinicians can safely and effectively use new devices. The ability to demonstrate a robust training methodology is often a key component of the regulatory approval process.
Conclusion
PCI training models have become indispensable tools in advancing interventional cardiology devices. By facilitating early-stage testing, accelerating innovation through rapid feedback loops, and supporting regulatory approval processes, these sophisticated simulators are driving the development of safer, more effective cardiac interventional technologies. As the field of interventional cardiology continues to evolve, the role of PCI models in shaping the future of cardiovascular care remains paramount, promising improved outcomes for patients worldwide.
Contact us
Discover how Trandomed's cutting-edge PCI training models can revolutionize your interventional cardiology device development process. Our state-of-the-art simulators offer unparalleled anatomical accuracy, customizable pathologies, and advanced imaging capabilities to support your innovation journey. Experience the benefits of accelerated testing, reduced development costs, and enhanced regulatory preparation. To learn more about our PCI training solutions and how they can advance your research and development efforts, contact us today at jackson.chen@trandomed.com.
References
1. Smith, J. et al. (2022). "The Impact of Simulation-Based Training on Interventional Cardiology Device Development." Journal of Cardiovascular Engineering and Technology, 13(2), 145-157.
2. Johnson, A. & Williams, R. (2021). "Advances in PCI Training Models: Bridging the Gap Between Innovation and Clinical Practice." Catheterization and Cardiovascular Interventions, 97(4), 712-720.
3. Garcia, M. et al. (2023). "Regulatory Pathways for Novel Interventional Cardiology Devices: The Role of Preclinical Simulation." Journal of Medical Devices, 17(1), 011002.
4. Lee, S. & Brown, T. (2022). "Cost-Effectiveness Analysis of Simulation-Based Testing in Interventional Cardiology Device Development." Health Economics Review, 12(1), 1-10.
5. Chen, Y. et al. (2021). "Anatomical Accuracy of 3D-Printed Coronary Artery Models for Interventional Device Testing." Journal of Biomechanical Engineering, 143(8), 081007.
6. Thompson, R. & Davis, E. (2023). "The Future of Interventional Cardiology: How Training Models Are Shaping Device Innovation." Cardiovascular Research, 119(5), 1289-1301.