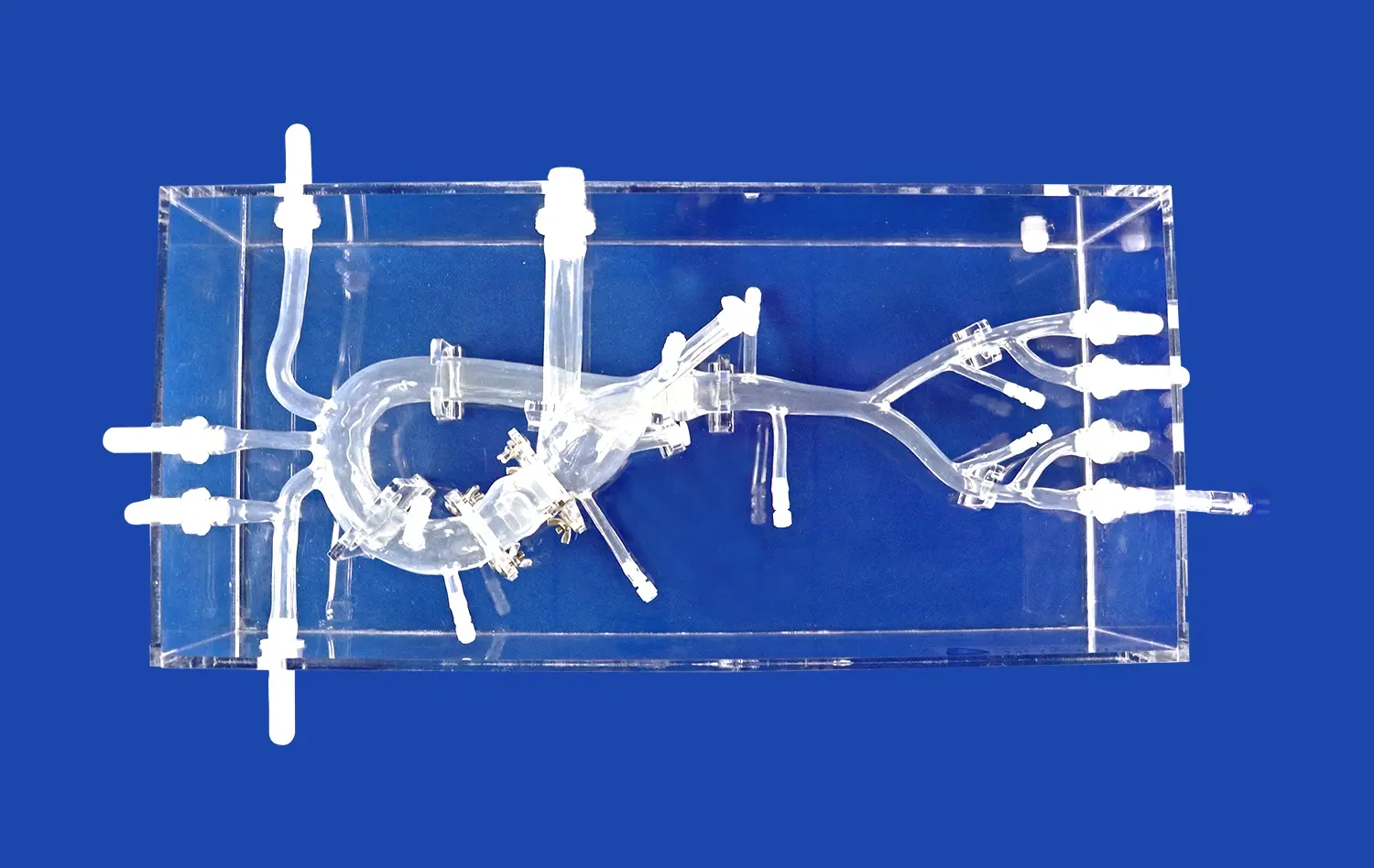
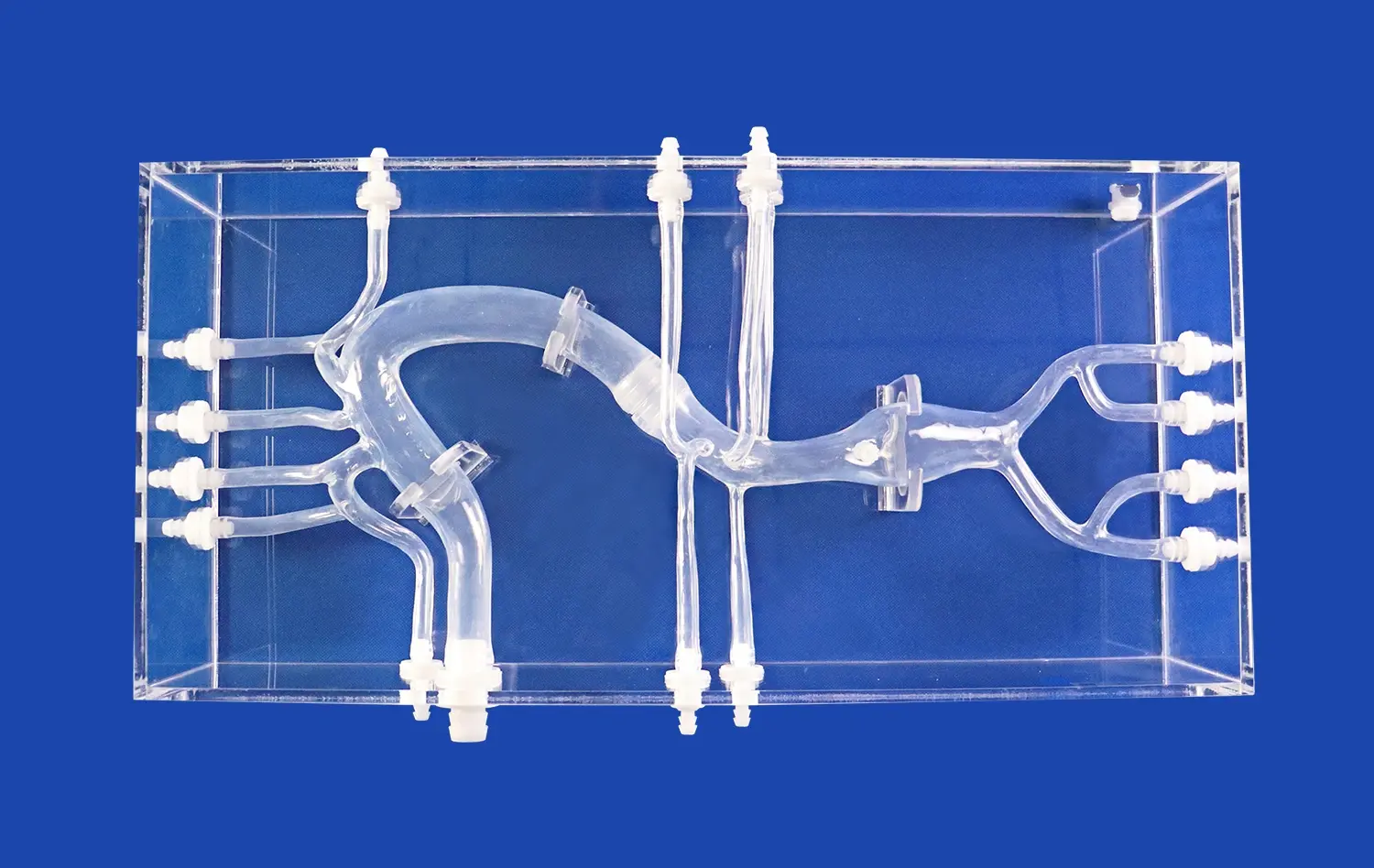
Why Are Pulmonary Artery Models Essential for Device Testing?
Anatomical Accuracy and Realism
High-fidelity pulmonary artery models offer unparalleled anatomical accuracy, replicating the intricate branching patterns, vessel dimensions, and tissue properties of the human pulmonary vasculature. This level of realism allows device manufacturers to test their products in conditions that closely mimic the complexities of the actual cardiovascular system. By incorporating details such as vessel tortuosity, bifurcations, and varying lumen diameters, these models enable developers to assess how their devices navigate and perform within challenging anatomical landscapes.
Customizable Pathologies
Advanced pulmonary vessel simulators can be customized to represent specific pathological conditions, such as pulmonary embolism, arterial stenosis, congenital malformations, pulmonary artery model. This flexibility allows device manufacturers to evaluate their products across a spectrum of disease states and anatomical variations. By testing interventional tools in models that replicate real patient scenarios, companies can refine their designs to address the unique challenges posed by different pulmonary vascular pathologies.
Repeatability and Standardization
Unlike cadaveric specimens or animal models, synthetic pulmonary artery models offer consistent, repeatable testing conditions. This standardization is crucial for comparing different device iterations, assessing performance improvements, and conducting controlled studies. The ability to replicate exact anatomical configurations across multiple tests enables more reliable data collection and analysis, supporting evidence-based design decisions throughout the development process.
Controlled Environment for Pre-Clinical Device Evaluation
Simulating Procedural Techniques
Pulmonary artery models serve as invaluable platforms for simulating various interventional procedures. Device manufacturers can use these models to replicate the entire journey of their products, from vascular access points to target treatment areas within the pulmonary arteries. This comprehensive simulation allows for the evaluation of device trackability, pushability, and maneuverability under realistic conditions. By observing how their devices interact with anatomical structures and overcome potential obstacles, developers can identify areas for improvement and optimize procedural techniques.
Assessing Device-Tissue Interactions
Advanced pulmonary vessel simulators often incorporate materials, specifically for constructing pulmonary artery models, that mimic the mechanical properties of vascular tissues. This feature enables manufacturers to assess how their devices interact with vessel walls, evaluate the risk of trauma or perforation, and optimize device designs for gentle navigation through delicate pulmonary vasculature. By studying these device-tissue interactions in a controlled setting, companies can refine their products to minimize the risk of complications and enhance overall safety profiles.
Imaging Compatibility Testing
Many interventional procedures rely heavily on imaging guidance, such as fluoroscopy or ultrasound. Pulmonary artery models designed with radiopaque materials or echogenic properties allow device manufacturers to evaluate the visibility and tracking of their products under various imaging modalities. This testing is crucial for ensuring that devices can be accurately visualized and positioned during procedures, contributing to improved procedural outcomes and reduced radiation exposure for both patients and clinicians.
Reducing Clinical Risks Through Iterative Simulation Studies
Rapid Prototyping and Design Refinement
The use of pulmonary artery models in the development process enables rapid prototyping and iterative design improvements. Manufacturers can quickly test multiple device variations, assess their performance in realistic anatomical settings, and make data-driven refinements. This accelerated development cycle not only reduces time-to-market but also allows for thorough optimization of device characteristics before progressing to more costly and time-consuming clinical trials.
Identifying Potential Failure Modes
Simulation studies using anatomically accurate pulmonary vessel models help identify potential failure modes or limitations of interventional devices. By subjecting products to a wide range of anatomical variations and procedural scenarios, manufacturers can uncover unforeseen challenges or weaknesses in their designs. This proactive approach to risk assessment allows companies to address potential issues early in the development process, ultimately leading to safer and more reliable devices.
Training and Procedural Optimization
Beyond product development, pulmonary artery models play a crucial role in training healthcare professionals and optimizing procedural techniques. Device manufacturers can use these models to develop comprehensive training programs, allowing clinicians to gain hands-on experience with new technologies in a risk-free environment. This educational aspect not only enhances the safety and efficacy of interventional procedures but also facilitates the adoption of novel devices in clinical practice.
Conclusion
Pulmonary artery models have become indispensable tools in the development of interventional devices for cardiovascular treatments. By providing anatomically accurate, customizable, and repeatable testing environments, these models enable manufacturers to refine their products, assess performance, and mitigate risks before clinical implementation. The ability to simulate complex anatomies, pathologies, and procedural techniques in a controlled setting accelerates innovation while prioritizing patient safety. As the field of interventional cardiology continues to advance, the role of high-fidelity pulmonary vessel simulators in driving product development and improving clinical outcomes will only continue to grow.
Contact Us
For cutting-edge pulmonary artery models that can revolutionize your interventional device development process, look no further than Trandomed. Our advanced 3D-printed silicone simulators offer unparalleled anatomical accuracy and customization options to meet your specific research and testing needs. Experience the benefits of working with a leader in medical simulation technology. Contact us today at jackson.chen@trandomed.com to explore how our pulmonary artery models can accelerate your innovation and enhance patient outcomes.
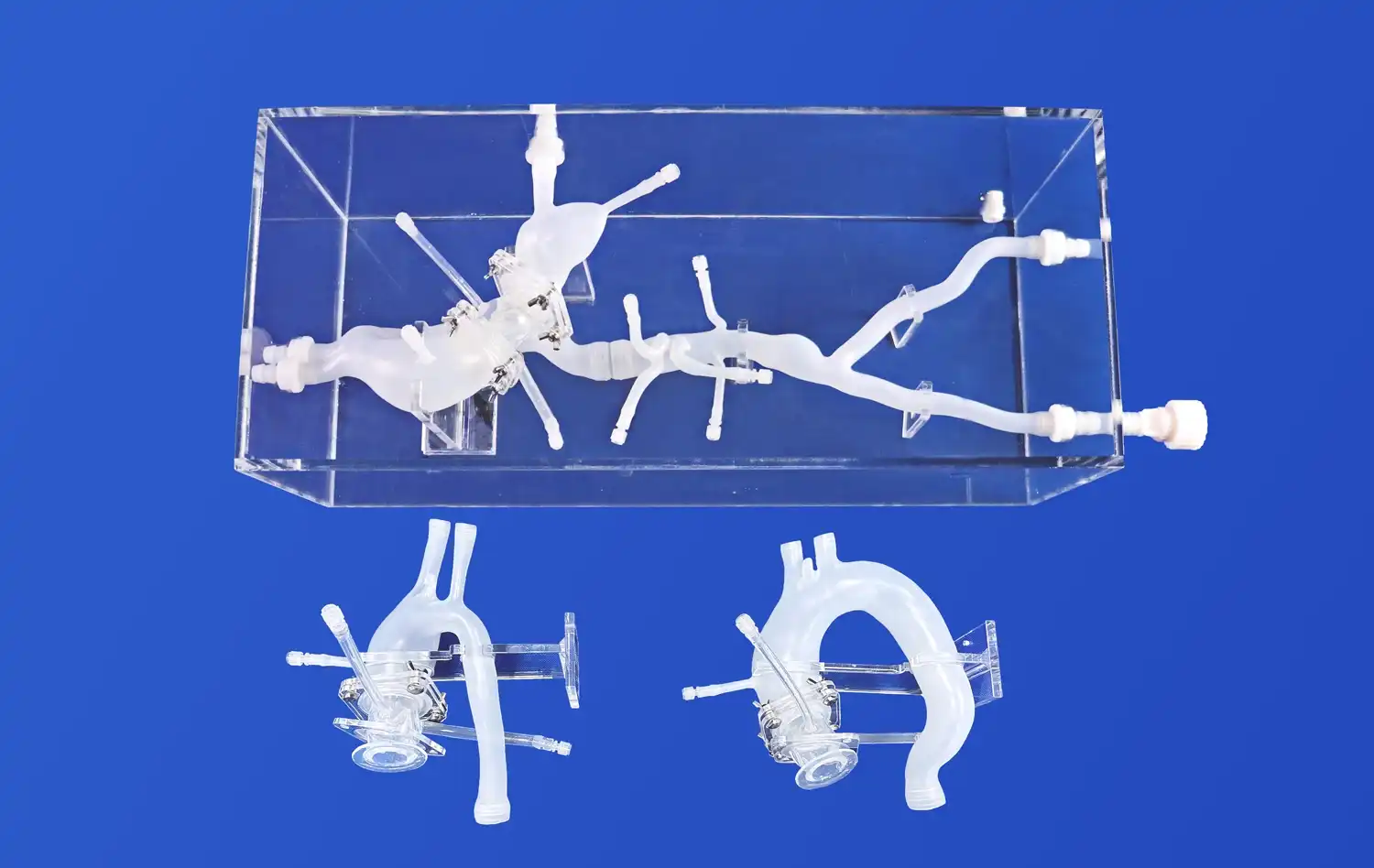
_1736216292718.webp)
 (SJ001D)_1734504338727.webp)
1_1732869849284.webp)