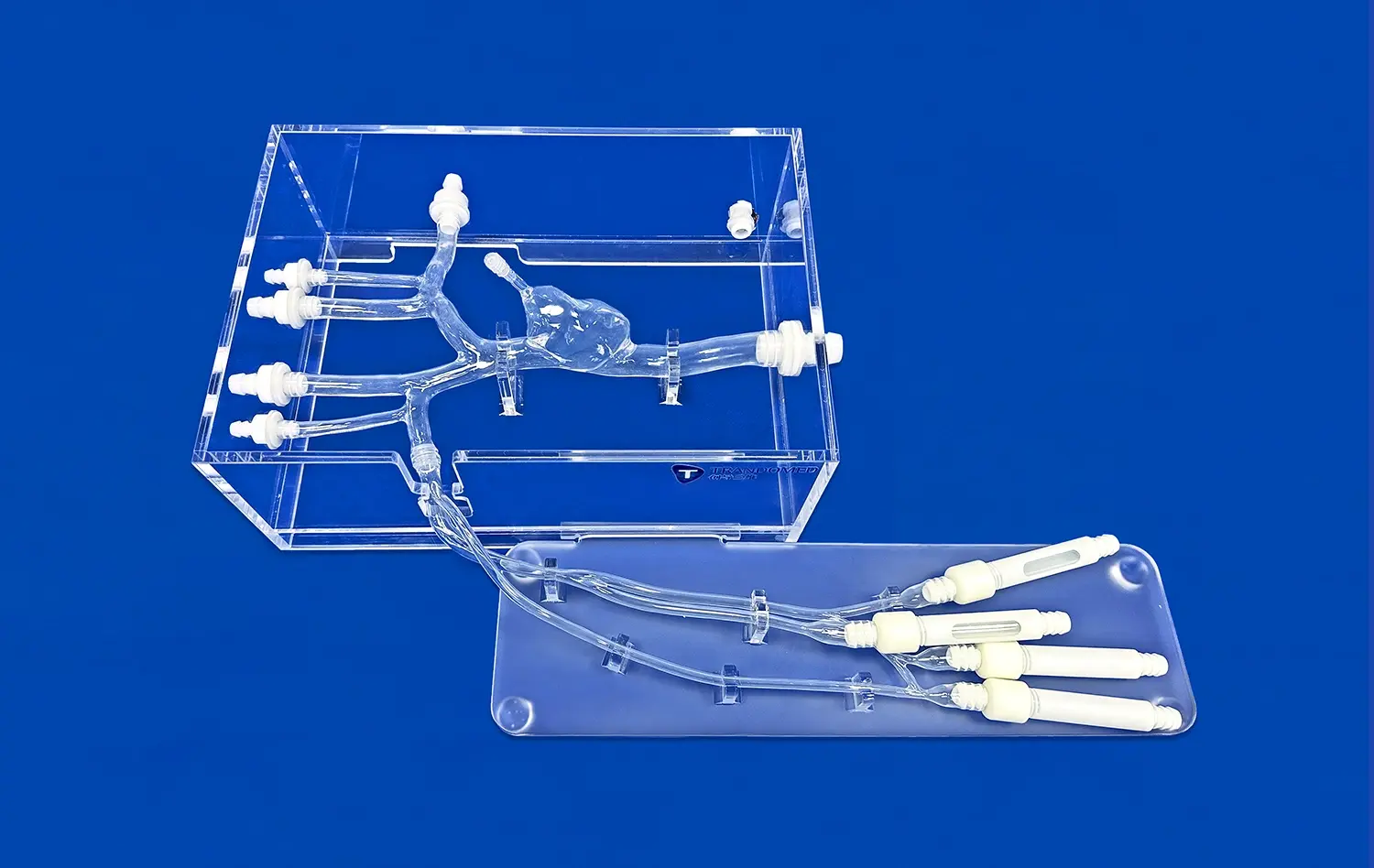
Evaluating Catheter, Stent, and Guide Wire Performance
Realistic Vessel Anatomy for Precise Testing
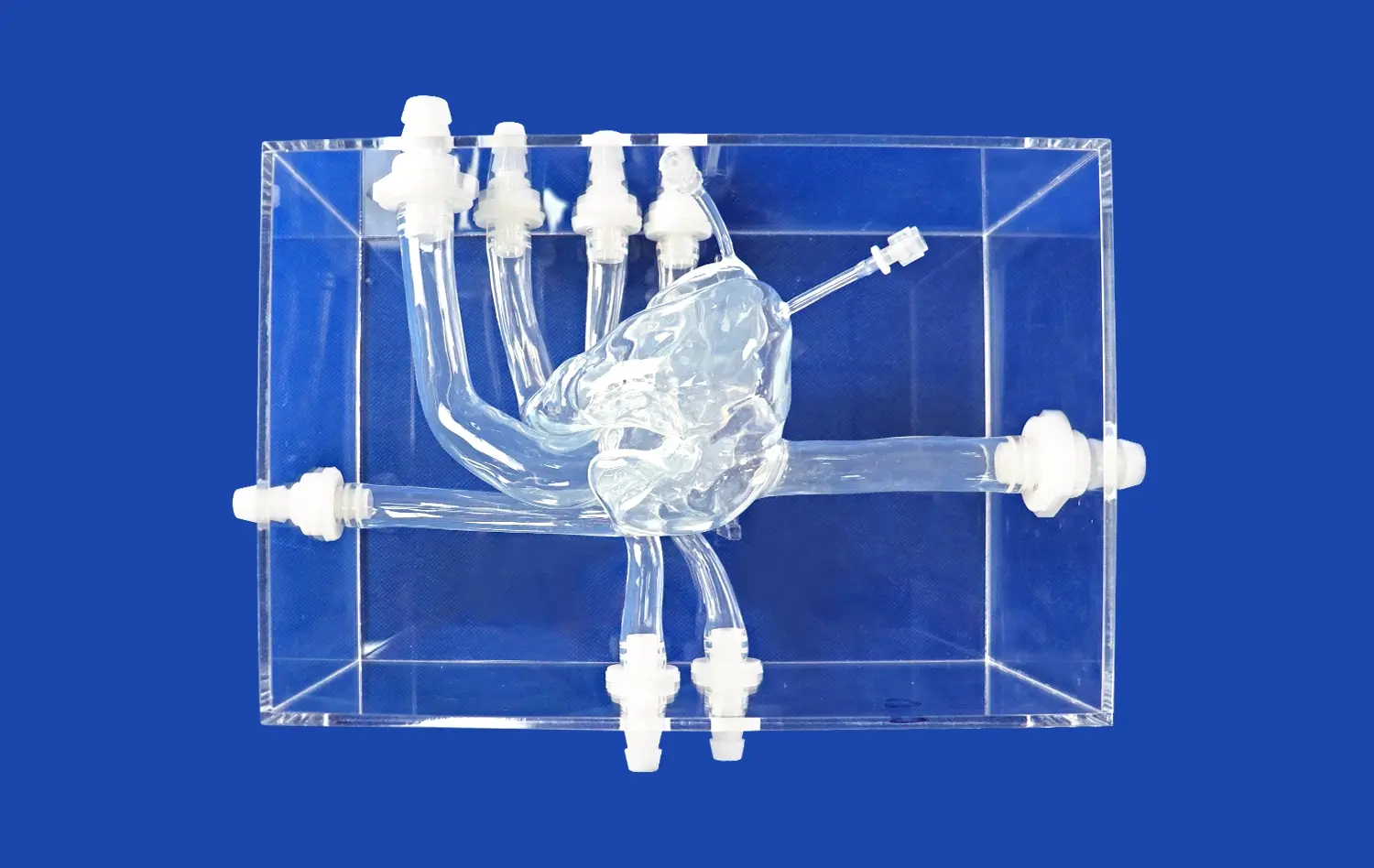
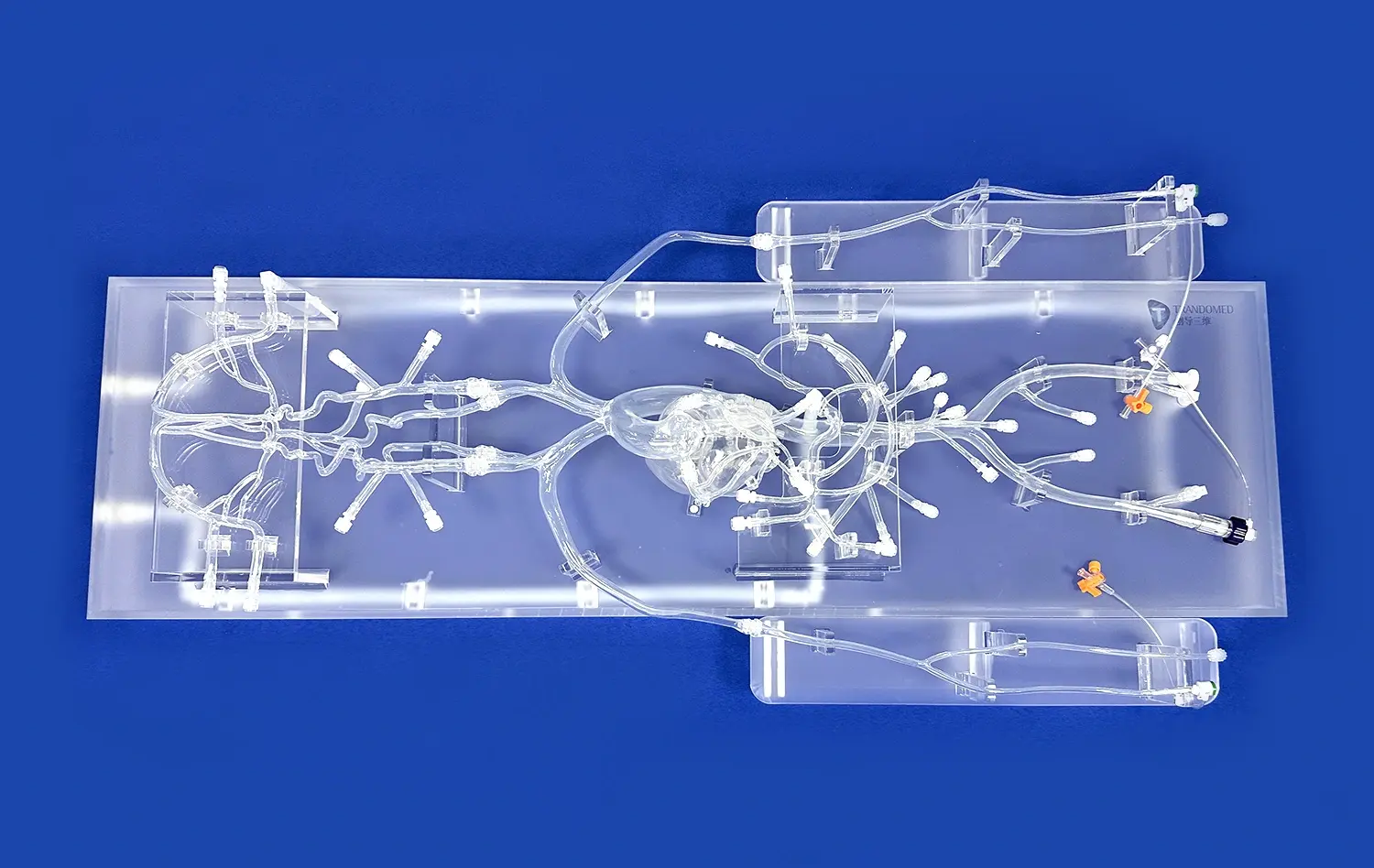
The Full Body Artery model's intricate design, based on real human CT and MRI data, offers an unparalleled testing environment for neurovascular devices. Its anatomically accurate representation of the arterial system, from the femoral entry point to the intracranial vessels, allows developers to assess device performance in conditions that closely mimic real-world scenarios. This level of realism is crucial for evaluating the navigation capabilities of catheters and guide wires, as well as the deployment characteristics of stents in various vessel geometries.
Simulating Challenging Pathologies
One of the key advantages of the full body artery model is its incorporation of pathological features, such as aneurysms and stenotic lesions. These inclusions enable developers to test their devices in complex anatomical situations, providing valuable insights into how catheters, stents, and guide wires perform when confronted with challenging vascular abnormalities. By simulating these conditions, the model helps identify potential issues and areas for improvement in device design and functionality.
Comprehensive Performance Evaluation
The Full Body Artery simulator facilitates a holistic assessment of neurovascular devices. Researchers can evaluate various performance metrics, including device trackability, pushability, and torque response throughout the entire vascular pathway. This comprehensive evaluation helps in identifying any limitations or areas of improvement in device design, ultimately leading to the development of more effective and safer neurovascular interventional tools.
Reducing Risk Through Preclinical Simulation
Enhancing Safety Protocols
By utilizing the Full Body Artery model in preclinical testing, medical device manufacturers can significantly enhance their safety protocols. The simulator allows for repeated testing of devices under various conditions, helping to identify potential risks or complications that may arise during clinical use. This thorough evaluation process contributes to the development of more robust safety measures and guidelines for neurovascular interventions.
Minimizing Clinical Trial Risks
The use of advanced simulation tools like the full body artery model can substantially reduce the risks associated with clinical trials. By thoroughly testing and refining devices in a realistic preclinical setting, manufacturers can address potential issues before human trials begin. This approach not only enhances patient safety but also increases the likelihood of successful clinical outcomes, potentially streamlining the regulatory approval process.
Training and Skill Development
Beyond device testing, the Full Body Artery model serves as an invaluable tool for training interventional neurologists and vascular surgeons. The simulator provides a risk-free environment for practitioners to hone their skills in complex neurovascular procedures, such as aneurysm coiling or stent placement. This hands-on experience with realistic anatomy and pathologies contributes to improved procedural competence and reduced risks in actual clinical settings.
Accelerating Innovation in Neurovascular Interventions
Rapid Prototyping and Iteration
The Full Body Artery model's versatility and accuracy make it an ideal platform for rapid prototyping and iteration in neurovascular device development. Manufacturers can quickly test new design concepts, materials, and technologies in a realistic setting, allowing for faster refinement and optimization of devices. This accelerated development cycle can significantly reduce time-to-market for innovative neurovascular interventions, benefiting both patients and the medical industry.
Advancing Treatment Strategies
By providing a comprehensive simulation environment, the full body artery model enables researchers to explore and develop novel treatment strategies for neurovascular conditions. The ability to test new approaches in a controlled, realistic setting fosters innovation in procedural techniques and device applications. This can lead to the development of more effective and less invasive treatment options for conditions such as intracranial aneurysms, arteriovenous malformations, and ischemic stroke.
Collaborative Research and Development
The Full Body Artery simulator serves as a valuable tool for collaborative research and development efforts. Its standardized platform allows for consistent testing and comparison of different devices and techniques across research institutions and industry partners. This collaborative approach can accelerate the pace of innovation in neurovascular interventions by facilitating knowledge sharing and collective problem-solving in the field.
Conclusion
The full body artery model represents a significant advancement in neurovascular device development and testing. Its realistic anatomical features, including pathological elements, provide an unparalleled platform for evaluating catheter, stent, and guide wire performance. By enabling thorough preclinical simulation, the model contributes to risk reduction in device development and clinical trials. Furthermore, it accelerates innovation in neurovascular interventions by facilitating rapid prototyping, advancing treatment strategies, and fostering collaborative research. As the field of interventional neurology continues to evolve, tools like the Full Body Artery model will play a crucial role in shaping the future of neurovascular care and improving patient outcomes.
Contact Us
As a leading 3D printed silicone medical simulators manufacturer, Trandomed is at the forefront of innovation in neurovascular device development. Our Full Body Artery model, crafted with precision using advanced 3D printing technology, offers unparalleled benefits for research, training, and device testing. With over 20 years of expertise and a commitment to customization, we provide tailored solutions to meet your specific needs. Experience the advantages of working with a trusted supplier and factory in the medical simulation industry. To learn more about how our Full Body Artery model can accelerate your neurovascular device development process, contact us at jackson.chen@trandomed.com.
References
Ionita, C. N., et al. (2020). "Challenges and limitations of patient-specific vascular phantom fabrication using 3D Printing." In Proceedings of SPIE--the International Society for Optical Engineering (Vol. 11315). NIH Public Access.
Suzuki, Y., et al. (2019). "Usefulness of a novel 3D-printed vascular model for preoperative simulation of carotid artery stenting." Neurologia medico-chirurgica, 59(11), 458-464.
Mashiko, T., et al. (2018). "Development of three-dimensional hollow elastic model for cerebral aneurysm clipping simulation enabling rapid and low cost prototyping." World neurosurgery, 109, 691-696.
Chueh, J. Y., et al. (2020). "Novel distal embolic protection technology: the EmboTrap." Interventional Neurology, 8(2-6), 171-186.
Toth, G., et al. (2018). "Current and potential future use of laser interstitial thermal therapy in the treatment of brain tumors." CNS oncology, 7(2), CNS20.
Spiotta, A. M., et al. (2018). "The COMPASS Trial: A Direct Aspiration First Pass Technique for Acute Ischemic Stroke." Journal of neurointerventional surgery, 10(Suppl 1), i3-i7.
_1732863962417.webp)