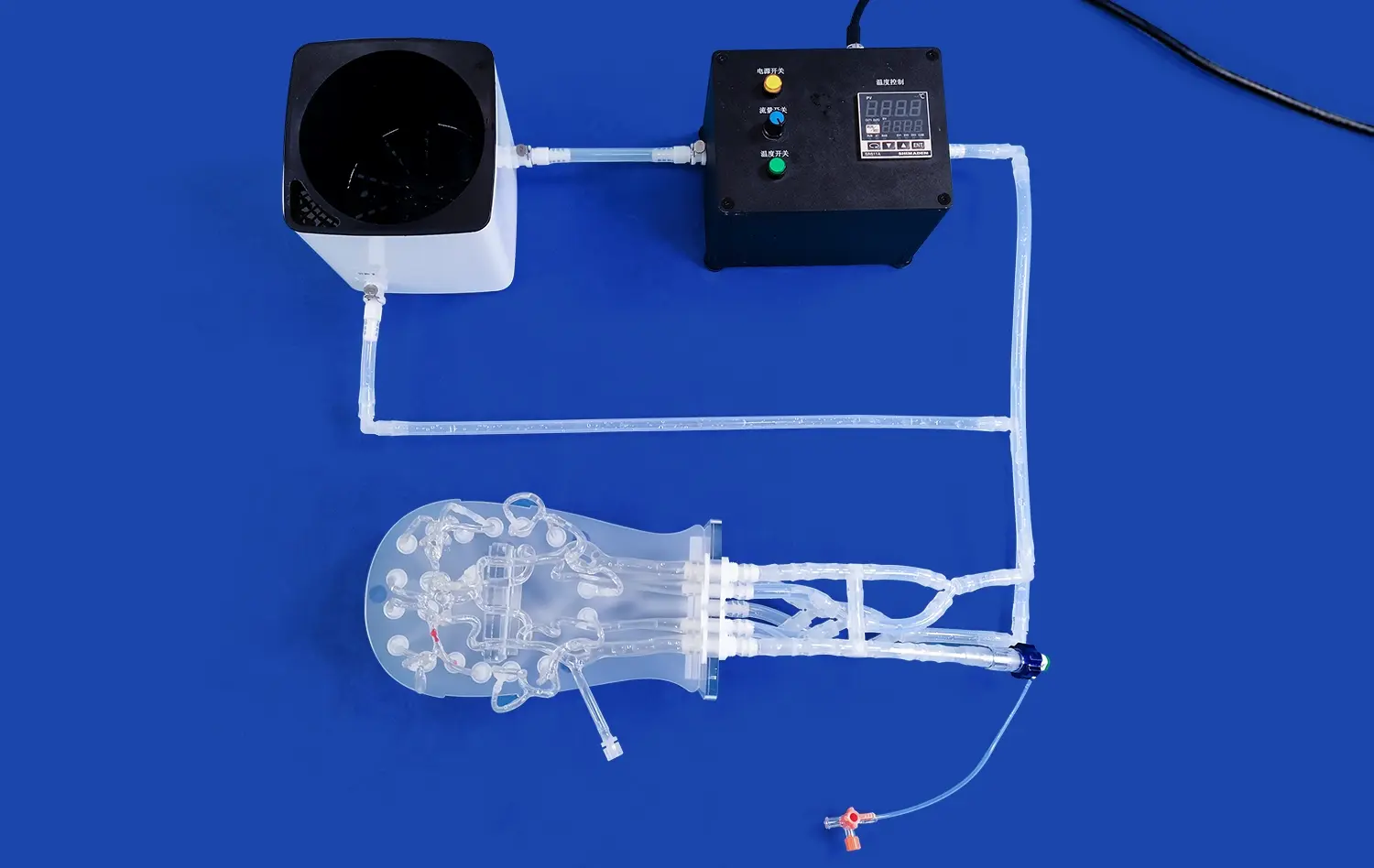
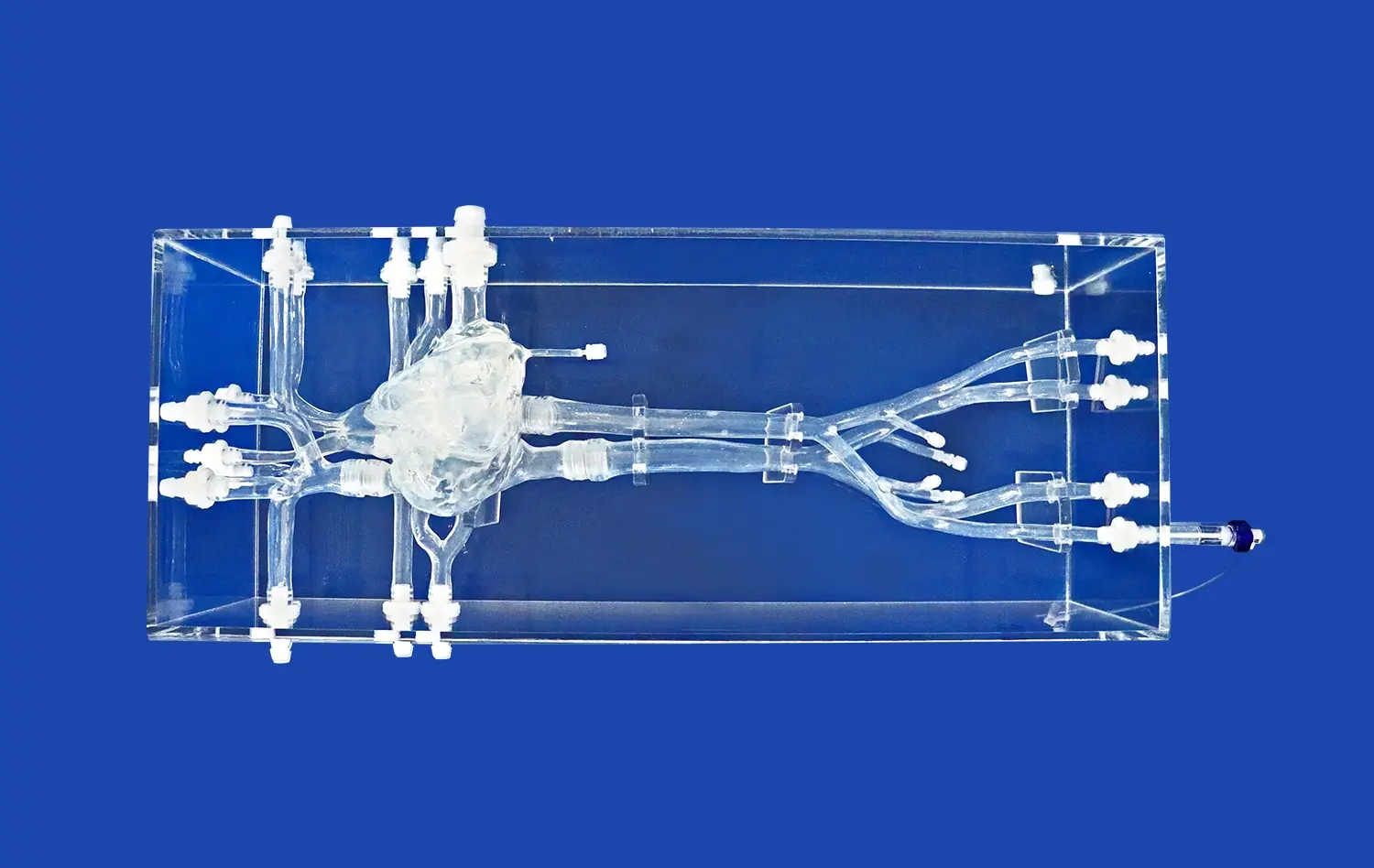
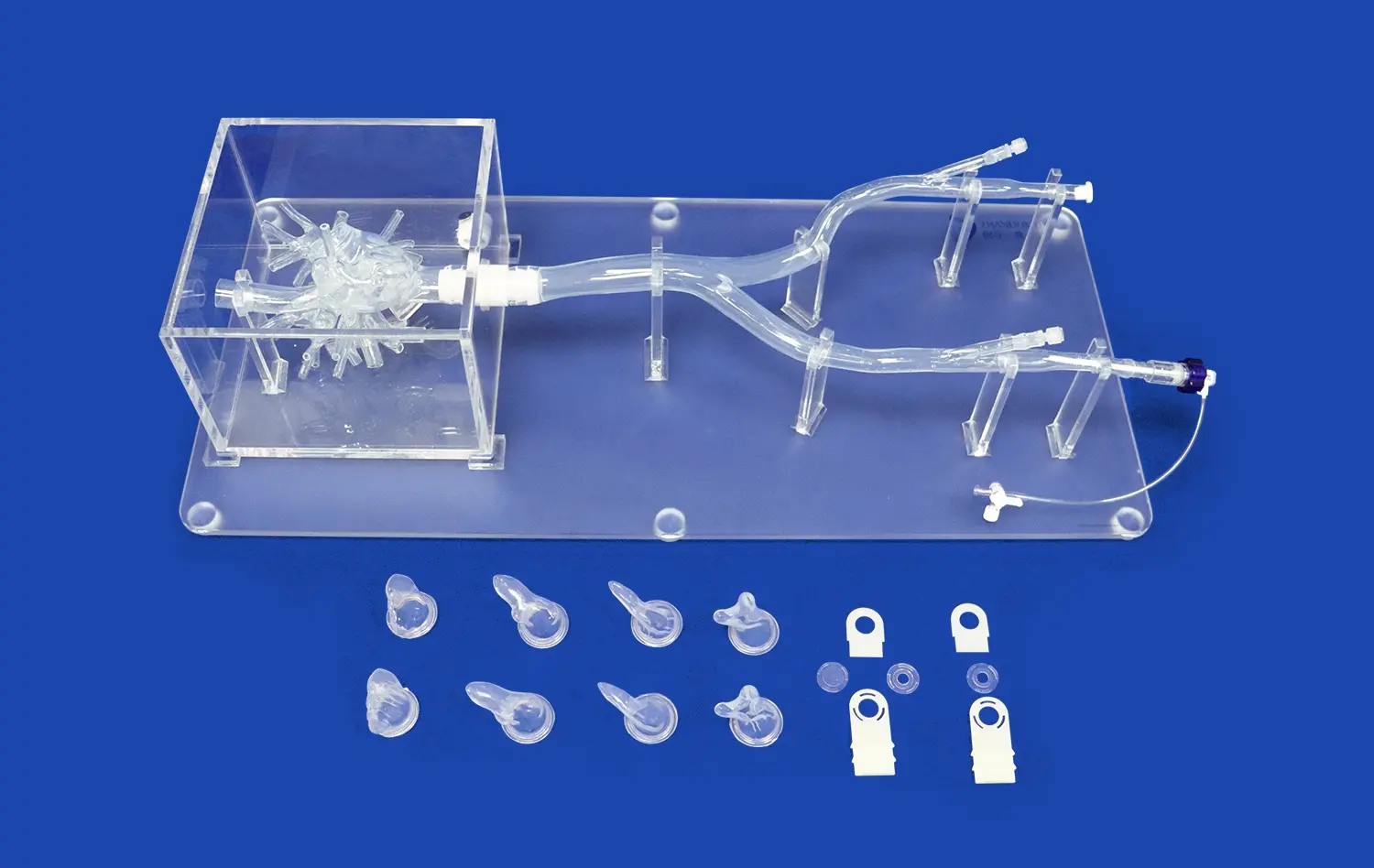
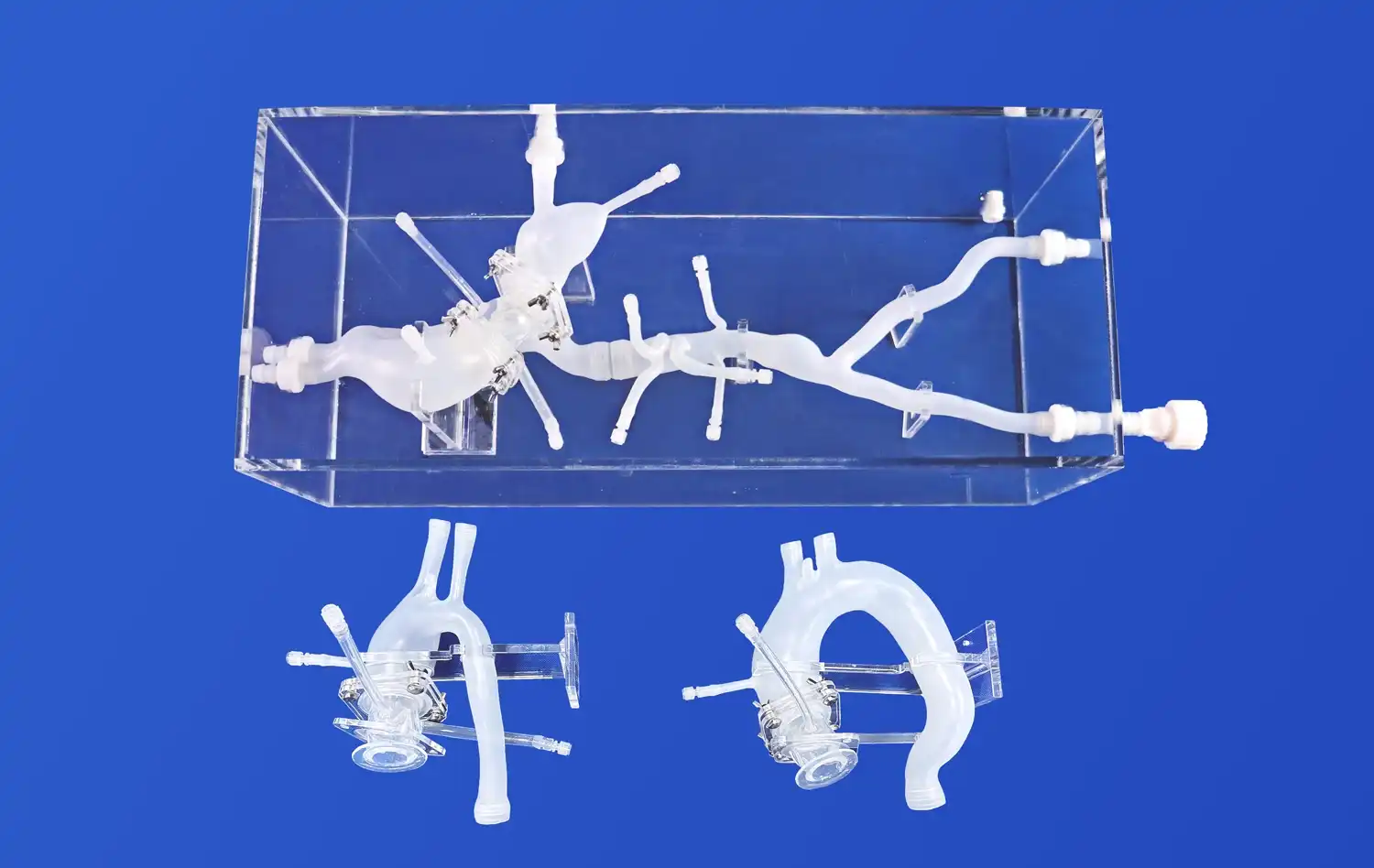
Heart models have revolutionized the landscape of medical device development, offering unprecedented opportunities for innovation and refinement in cardiovascular care. These anatomically accurate replicas serve as invaluable tools for researchers, engineers, and clinicians engaged in creating cutting-edge cardiac interventional devices. By providing a realistic platform for testing and validation, heart models significantly accelerate the product development cycle while enhancing safety and efficacy.
From intricate coronary stents to sophisticated valve replacement systems, these models enable developers to visualize, prototype, and iterate designs in a controlled environment that closely mimics real-world conditions. This approach not only streamlines the development process but also contributes to reducing animal testing and minimizing risks associated with early-stage human trials. As the medical device industry continues to evolve, the role of high-fidelity heart models in shaping innovative cardiovascular solutions becomes increasingly pivotal.
How Do Heart Models Support Device Prototyping and Testing?
Anatomical Accuracy and Material Fidelity
Heart models designed for medical device development boast exceptional anatomical precision, replicating the intricate structures of the human heart with remarkable detail. These models, often crafted using advanced 3D printing techniques and specialized materials, accurately represent the chambers, valves, and vasculature of the heart. The use of silicone with specific Shore hardness ratings, such as Shore 40A, allows developers to simulate the elasticity and tactile properties of cardiac tissues. This level of fidelity is crucial for assessing how devices interact with heart structures, ensuring that prototypes can be tested under conditions that closely approximate in vivo environments.
Simulation of Physiological Conditions
Modern heart models go beyond static representations, incorporating dynamic elements that simulate physiological conditions. These may include pulsatile flow systems that mimic blood circulation, adjustable pressure settings to represent various cardiac states, and even simulated pathologies. Such features allow device developers to test their prototypes under a range of conditions, from normal heart function to specific disease states. This capability is invaluable for evaluating device performance, durability, and potential failure modes across diverse scenarios that may be encountered in clinical use.
Transparency and Visualization
Many advanced heart models, like the Heart Model with Coronary (XXK002DJ), are designed with transparency in mind. Encased in clear acrylic boxes, these models offer unobstructed views of internal structures and device interactions. This transparency is crucial for visual assessment during prototyping and testing phases. Developers can directly observe how devices navigate through vessels, deploy within chambers, or interact with heart valves. This visual feedback is instrumental in identifying design flaws, optimizing device geometries, and refining deployment techniques early in the development process.
Simulated Deployment of Balloons, Stents, and Valves
Replicating Procedural Challenges
Heart models excel in simulating the deployment of various cardiovascular devices, offering a realistic environment for testing balloons, stents, and valve systems. These models are designed to replicate the challenges encountered during actual procedures, such as navigating tortuous vessels or positioning devices in specific cardiac locations. For instance, when testing coronary stents, developers can practice maneuvering through complex arterial networks, assessing deliverability, and evaluating expansion characteristics within stenotic segments. This level of simulation helps in refining device designs and deployment techniques before progressing to more advanced stages of testing.
Assessing Device-Tissue Interactions
One of the critical aspects of cardiovascular device development is understanding how these interventional tools interact with heart tissues. High-fidelity heart models provide a platform to assess these interactions in detail. For example, when simulating balloon angioplasty, developers can observe how different balloon designs affect vessel wall stress and potential dissection risks. Similarly, for valve deployment, these models allow for the evaluation of how prosthetic valves seat within native annuli, helping to optimize designs for better sealing and reduced paravalvular leakage. This insight is crucial for enhancing device safety and efficacy.
Iterative Design Refinement
The ability to repeatedly deploy and retrieve devices within heart models facilitates an iterative design process. Developers can quickly test multiple iterations of a device, making incremental improvements based on observed performance. This rapid prototyping and testing cycle is particularly valuable for fine-tuning aspects such as radial force in stents, leaflet design in prosthetic valves, or catheter flexibility in delivery systems. By identifying and addressing potential issues early in the development phase, companies can significantly reduce the time and resources required to bring a refined product to market.
Reducing Time-to-Market Through High-Fidelity Bench Testing
Accelerated Pre-Clinical Evaluation
High-fidelity heart models play a crucial role in accelerating the pre-clinical evaluation phase of medical device development. By providing a realistic platform for comprehensive bench testing, these models allow developers to conduct extensive evaluations that would traditionally require animal studies or early-stage human trials. This approach not only expedites the development timeline but also reduces the ethical concerns and costs associated with animal testing. Developers can perform numerous iterations and tests on their devices, refining designs and addressing potential issues before committing to more resource-intensive stages of development.
Enhanced Regulatory Submission Preparation
The data gathered from rigorous testing on advanced heart models significantly strengthens regulatory submissions. Detailed performance metrics, safety assessments, and efficacy data obtained through simulated use can provide regulatory bodies with comprehensive evidence of a device's potential in clinical applications. This robust pre-clinical data package can expedite the review process, potentially leading to faster approvals and market entry. Moreover, the ability to demonstrate device performance in anatomically accurate models can instill greater confidence in regulators regarding the safety and effectiveness of the proposed medical device.
Streamlined Clinical Trial Design
Insights gained from extensive testing on heart models can inform and optimize clinical trial designs. By identifying potential challenges or areas of concern during bench testing, developers can tailor their clinical protocols more effectively. This might involve refining patient selection criteria, adjusting procedural techniques, or focusing on specific performance metrics that showed promise in simulated environments. Such targeted approach to clinical trial design can lead to more efficient studies, potentially reducing the time and resources required to demonstrate clinical efficacy and safety.
Conclusion
The integration of advanced heart models in medical device development represents a significant leap forward in cardiovascular innovation. These high-fidelity simulations provide an unparalleled platform for prototyping, testing, and refining interventional devices, offering insights that were previously unattainable without extensive animal or human trials. By enabling more comprehensive bench testing, accelerating the development cycle, and enhancing regulatory submissions, heart models are instrumental in bringing safer, more effective cardiovascular devices to market faster. As technology continues to evolve, the role of these sophisticated models in shaping the future of cardiac care becomes increasingly indispensable.
Contact Us
At Trandomed, we're committed to advancing medical device development through our state-of-the-art heart models. Our anatomically accurate simulations, including the Heart Model with Coronary (XXK002DJ), offer unparalleled opportunities for testing and refining your cardiovascular devices. Experience the benefits of high-fidelity bench testing and accelerate your path to market. To learn more about how our heart models can support your development process or to discuss customization options, contact us at jackson.chen@trandomed.com. Let's innovate together for better cardiac care.
References
1. Russ, M., O'Hara, R., Setlur Nagesh, S. V., et al. (2021). Evaluation of 3D Printed Cardiovascular Models for Surgical Planning and Decision-Making in Congenital Heart Disease. JACC: Cardiovascular Imaging, 14(4), 836-849.
2. Vukicevic, M., Mosadegh, B., Min, J. K., & Little, S. H. (2017). Cardiac 3D Printing and its Future Directions. JACC: Cardiovascular Imaging, 10(2), 171-184.
3. Biglino, G., Capelli, C., Wray, J., et al. (2015). 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: feasibility and acceptability. BMJ Open, 5(4), e007165.
4. Ripley, B., Kelil, T., Cheezum, M. K., et al. (2016). 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. Journal of Cardiovascular Computed Tomography, 10(1), 28-36.
5. Valverde, I., Gomez-Ciriza, G., Hussain, T., et al. (2017). Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. European Journal of Cardio-Thoracic Surgery, 52(6), 1139-1148.
6. Wang, D. D., Gheewala, N., Shah, R., et al. (2014). Three-Dimensional Printing for Planning of Structural Heart Interventions. Interventional Cardiology Clinics, 3(3), 453-469.
_1735798438356.webp)