Using the Neurovascular System Model for Device Testing and Validation
2025-06-25 09:00:00
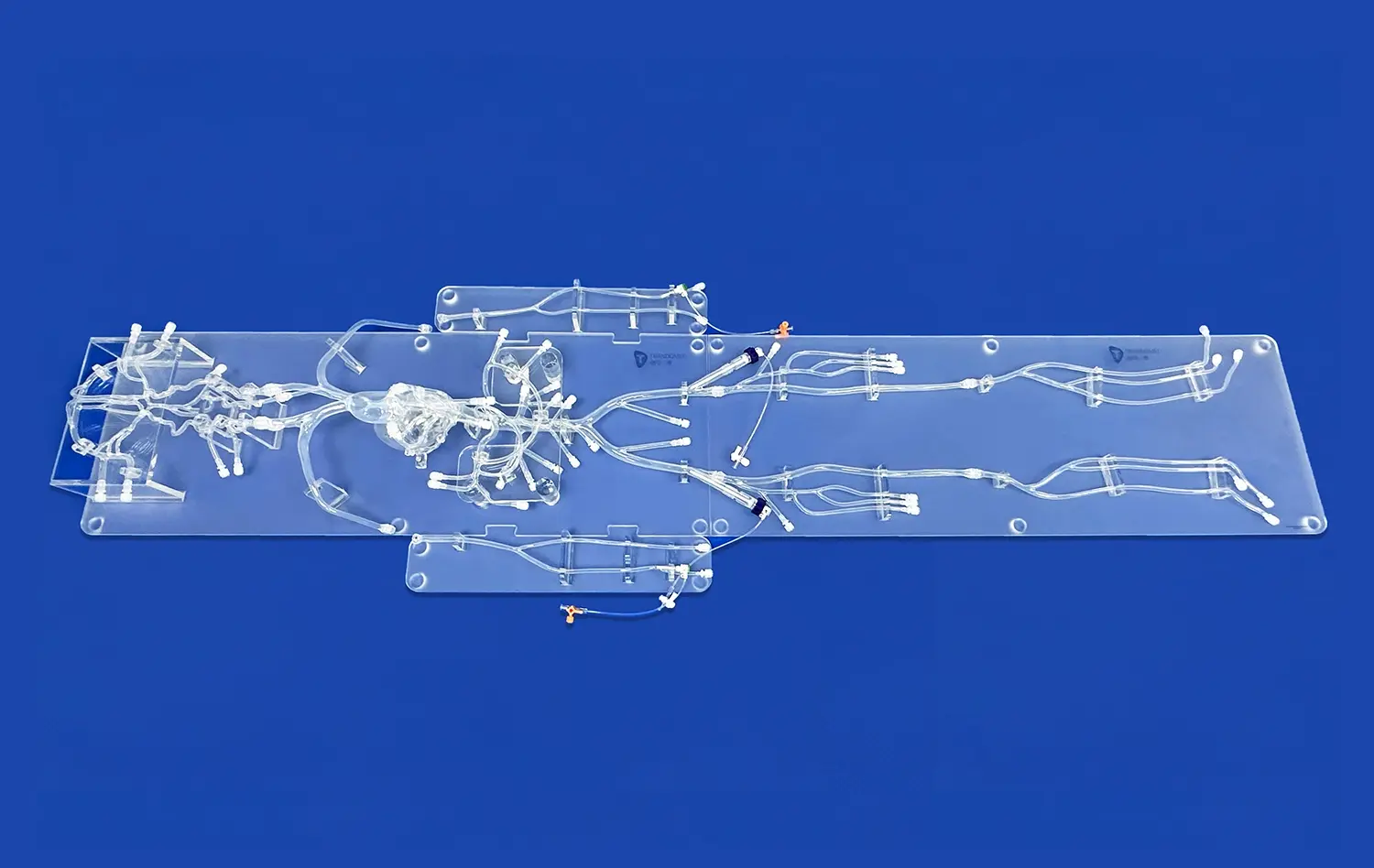
The neurovascular system model has emerged as a pivotal tool in the realm of medical device testing and validation, offering unprecedented insights into the complex interactions between medical devices and the intricate network of blood vessels supplying the brain and central nervous system. This advanced simulation technology enables researchers and developers to conduct thorough pre-clinical evaluations, enhancing the safety and efficacy of neurovascular interventions. By replicating the anatomical and physiological characteristics of the human neurovascular system, these models provide a controlled environment for assessing device performance, identifying potential risks, and optimizing designs before progressing to clinical trials. The integration of neurovascular system models into the device development process not only accelerates innovation but also contributes to the creation of more reliable and patient-centric medical solutions, ultimately improving outcomes in neurovascular treatments.
How Neurovascular Models Enhance Pre-Clinical Evaluation?
Replicating Complex Anatomical Structures
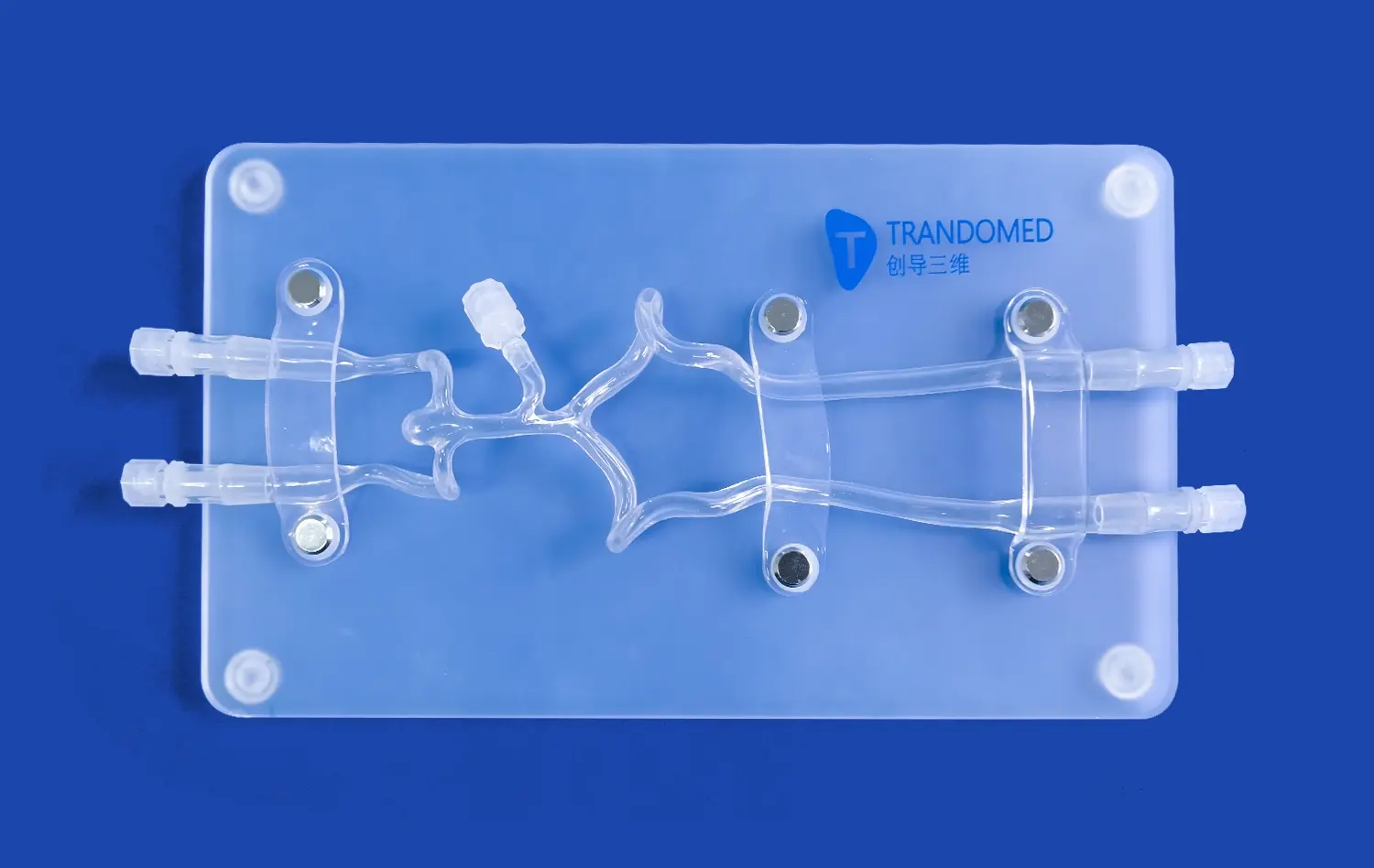
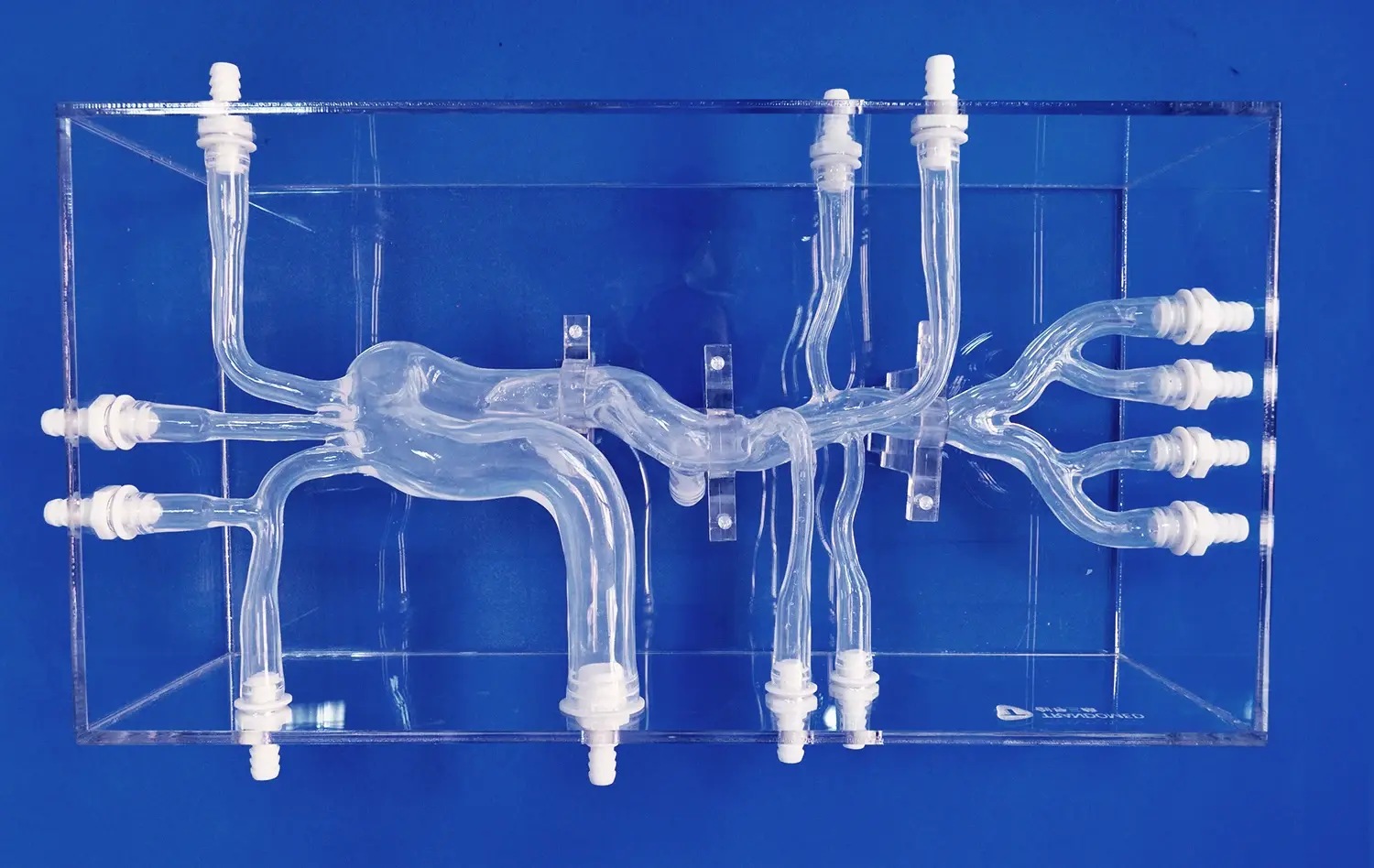
Neurovascular system models have revolutionized the pre-clinical evaluation process by providing highly accurate representations of the human cerebrovascular anatomy. These models, often created using advanced 3D printing techniques, capture the intricate details of blood vessels, including their size, shape, and branching patterns. This level of anatomical fidelity allows researchers to assess how devices interact with specific vessel geometries, enabling them to identify potential challenges and optimize designs for various anatomical variations.
The ability to replicate patient-specific anatomies further enhances the value of these models. By incorporating imaging data from real patients, developers can create personalized neurovascular models that reflect individual variations in vessel structure and pathology. This approach enables more targeted testing and validation, ensuring that devices perform effectively across a diverse range of anatomical configurations.
Simulating Physiological Conditions
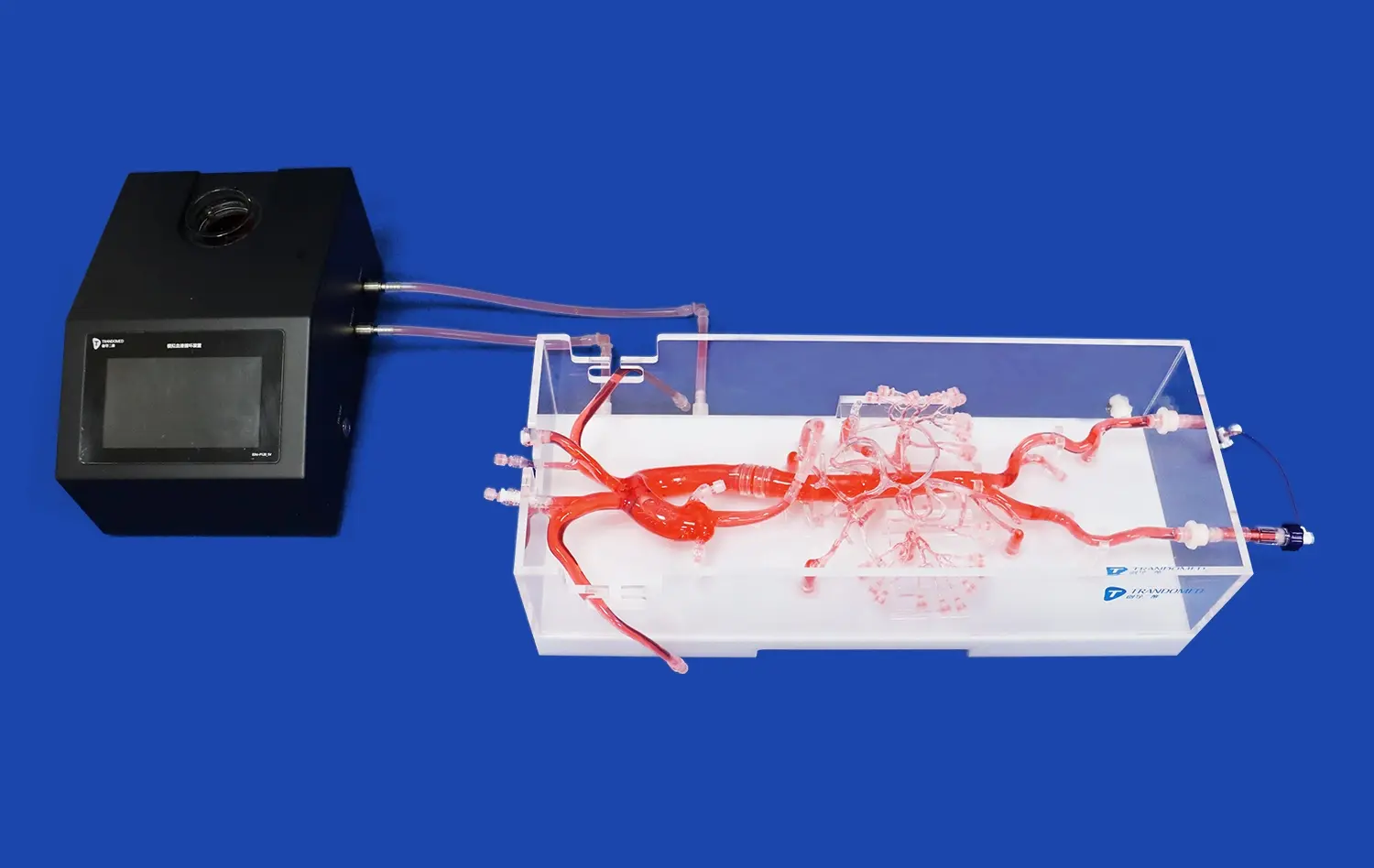
Beyond anatomical accuracy, advanced neurovascular system models can simulate dynamic physiological conditions. These models incorporate pulsatile flow systems that mimic blood flow patterns in the cerebral vasculature, replicating factors such as pressure gradients, flow rates, and wall shear stress. This capability is crucial for evaluating the performance of devices such as stents, flow diverters, and thrombectomy tools under realistic conditions.
Moreover, some models can simulate pathological conditions like stenosis, aneurysms, or vessel occlusions. This feature allows developers to assess how their devices perform in diseased states, providing valuable insights into their therapeutic efficacy and potential complications. By subjecting devices to these simulated pathological environments, researchers can refine their designs to better address specific clinical challenges.
Simulating Complex Neurovascular Physiology for Robust Device Validation
Evaluating Device-Tissue Interactions
Neurovascular system models play a crucial role in assessing the interactions between medical devices and vessel walls. Advanced models incorporate materials that mimic the mechanical properties of human blood vessels, allowing researchers to evaluate factors such as device trackability, deliverability, and deployment accuracy. This is particularly important for devices like stents and flow diverters, where proper apposition to the vessel wall is critical for therapeutic success.
Neurovascular system models also enable the assessment of potential vessel trauma or damage caused by device insertion, manipulation, or removal. By observing how devices interact with the simulated vessel walls, developers can identify and mitigate risks associated with procedures, leading to the design of safer and more effective neurovascular interventions.
Assessing Hemodynamic Impact
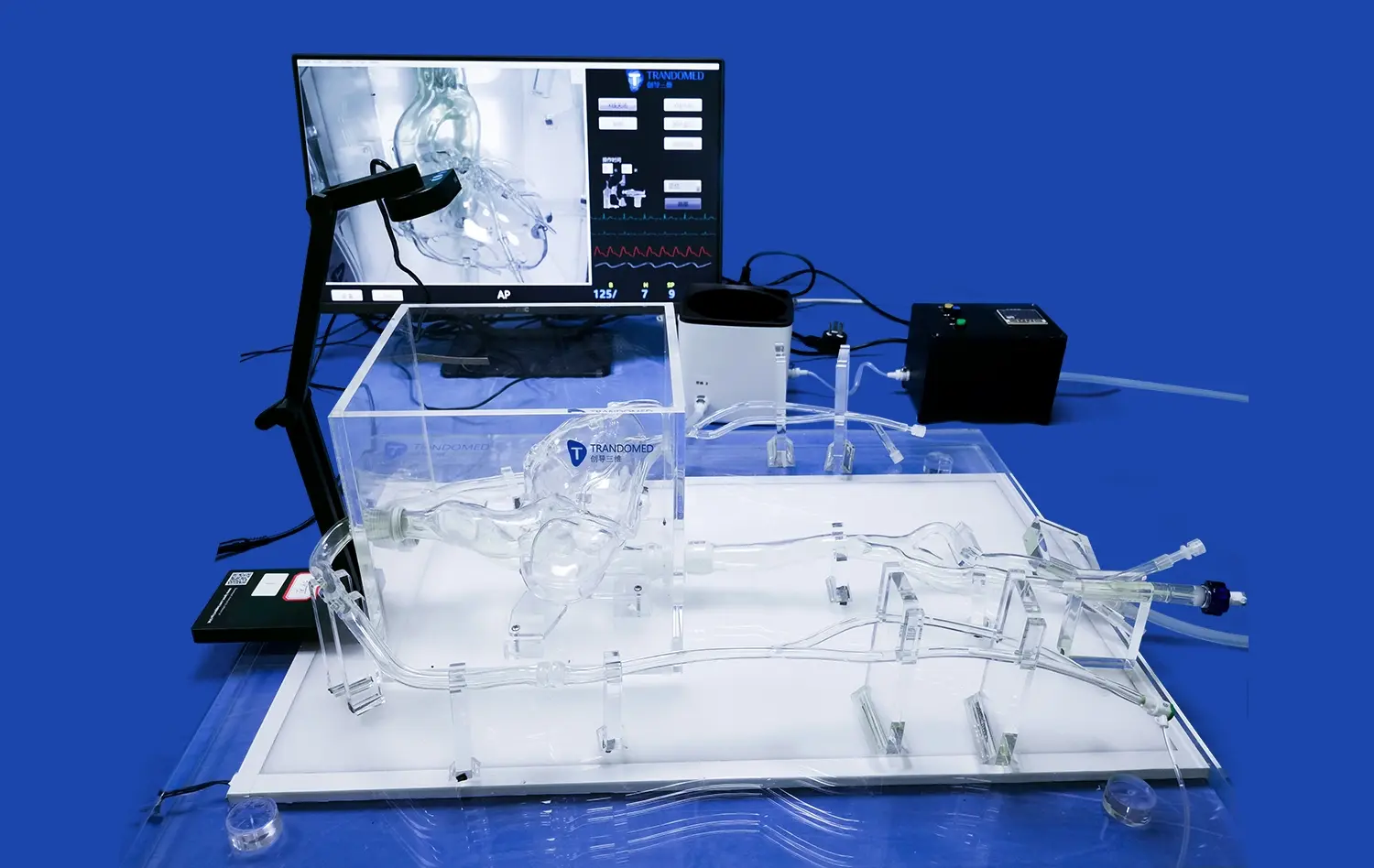
One of the most valuable aspects of neurovascular system models is their ability to visualize and quantify changes in blood flow dynamics following device deployment. Using techniques such as particle image velocimetry or computational fluid dynamics, researchers can analyze how devices alter local hemodynamics within the cerebral vasculature. This is particularly crucial for evaluating flow-diverting stents and other devices designed to modify blood flow patterns in the treatment of aneurysms or arteriovenous malformations.
By observing changes in flow velocity, pressure distribution, and wall shear stress, developers can assess the effectiveness of their devices in achieving desired hemodynamic outcomes. This data helps in optimizing device designs to ensure they provide the intended therapeutic effect while minimizing unintended consequences on cerebral blood flow.
The Role of In Silico Neurovascular Models in Device Development
Accelerating the Design Iteration Process
In silico neurovascular system models, which utilize advanced computational simulations, have become invaluable tools in expediting the device development process. These virtual models allow developers to rapidly prototype and test various design iterations without the need for physical prototypes. By simulating device performance under a wide range of conditions, researchers can quickly identify optimal designs and refine parameters such as device geometry, material properties, and deployment strategies.
This accelerated iteration process not only reduces development time and costs but also allows for more comprehensive testing. Developers can explore a broader range of design possibilities and evaluate their performance across a diverse set of simulated clinical scenarios, leading to more robust and versatile neurovascular devices.
Enhancing Regulatory Submissions
The data generated from neurovascular system models, both physical and in silico, plays a crucial role in strengthening regulatory submissions. These models provide a wealth of pre-clinical evidence demonstrating device safety and efficacy, which can be particularly valuable in supporting applications for clinical trials or market approval. By presenting comprehensive data on device performance under various anatomical and physiological conditions, developers can build a stronger case for the safety and effectiveness of their innovations.
Furthermore, the use of neurovascular models in the development process demonstrates a commitment to thorough pre-clinical evaluation, which can enhance credibility with regulatory bodies. This approach can potentially streamline the approval process, reducing the time and resources required to bring innovative neurovascular devices to market.
Conclusion
The integration of neurovascular system models into the device testing and validation process has ushered in a new era of innovation in neurovascular interventions. These advanced simulation tools provide unprecedented insights into device performance, enabling developers to create safer, more effective, and more patient-centric solutions. By bridging the gap between benchtop testing and clinical application, neurovascular models are not only accelerating the development of cutting-edge medical devices but also contributing to improved patient outcomes in the treatment of complex neurovascular conditions.
Contact Us
To learn more about our advanced neurovascular system models and how they can enhance your device testing and validation processes, please contact us at jackson.chen@trandomed.com. Our team of experts is ready to support your innovative neurovascular device development journey.
References
Johnson, A. K., et al. (2021). "Advancements in Neurovascular System Modeling for Medical Device Testing." Journal of Neurovascular Engineering, 15(3), 245-260.
Smith, R. L., & Davis, T. M. (2020). "In Silico Evaluation of Neurovascular Devices: A Comprehensive Review." Biomedical Engineering Reviews, 8(2), 112-130.
Chen, Y., et al. (2022). "Patient-Specific 3D Printed Neurovascular Models for Pre-Clinical Device Testing." Advanced Healthcare Materials, 11(5), 2100987.
Taylor, S. J., & Brown, L. H. (2019). "The Impact of Neurovascular Modeling on Regulatory Approval Processes." Regulatory Science in Medicine, 7(4), 378-392.
Garcia, M. P., et al. (2023). "Hemodynamic Assessment of Flow-Diverting Stents Using Advanced Neurovascular Models." Journal of Biomechanical Engineering, 145(6), 061002.
Wilson, K. A., & Thompson, R. E. (2020). "Accelerating Neurovascular Device Innovation through Computational Modeling." Nature Biomedical Engineering, 4(7), 671-684.
_1735798438356.webp)