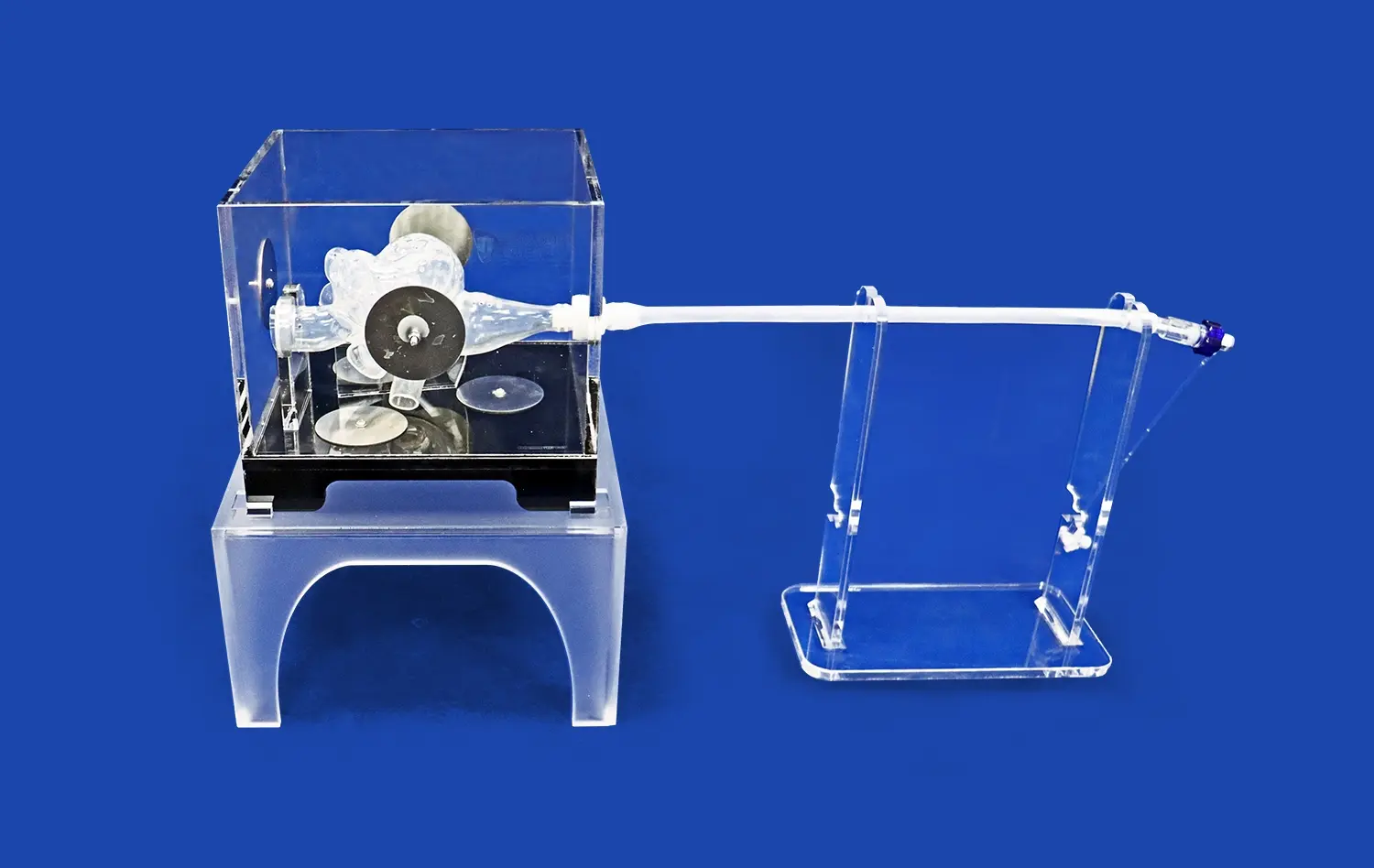
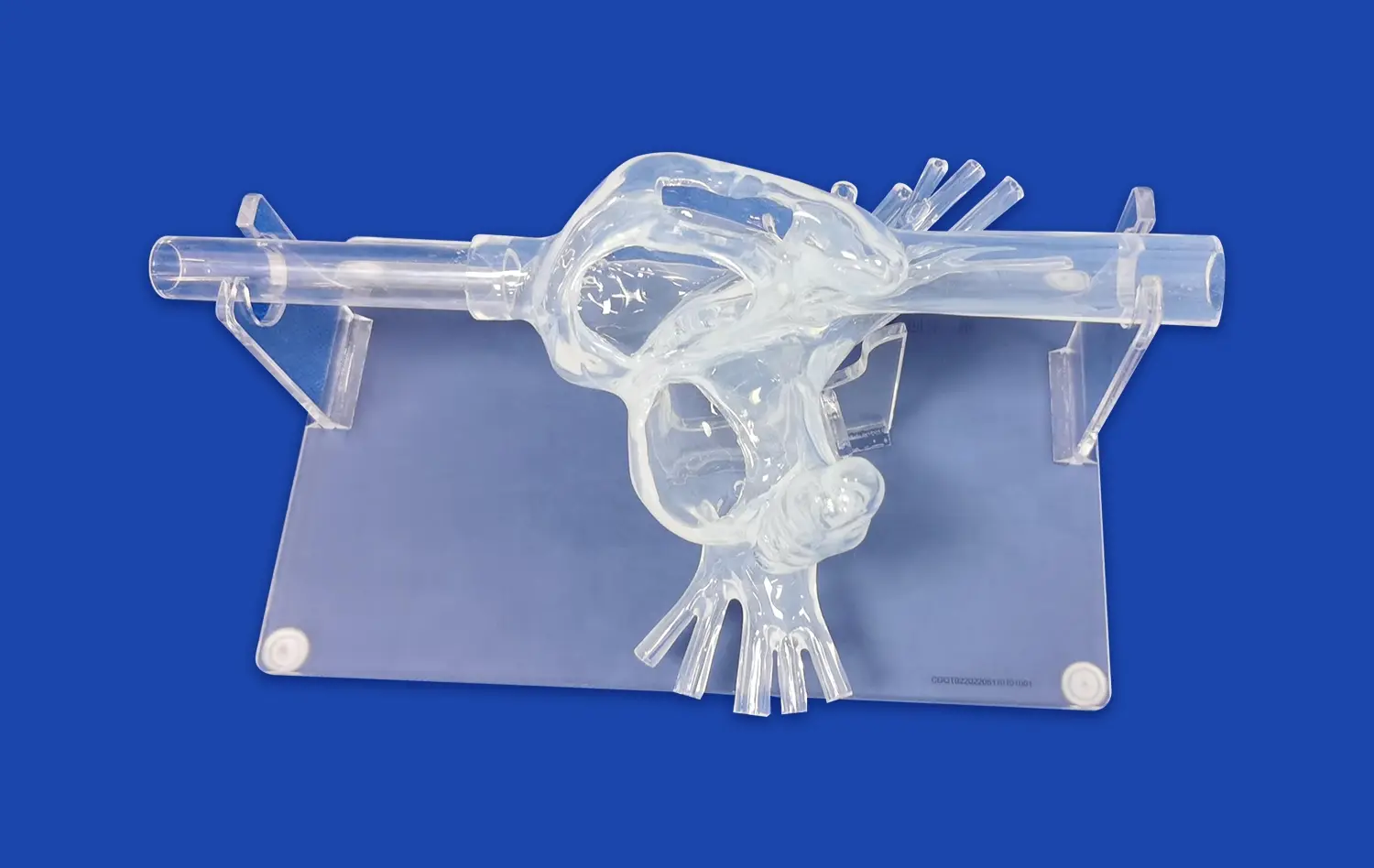
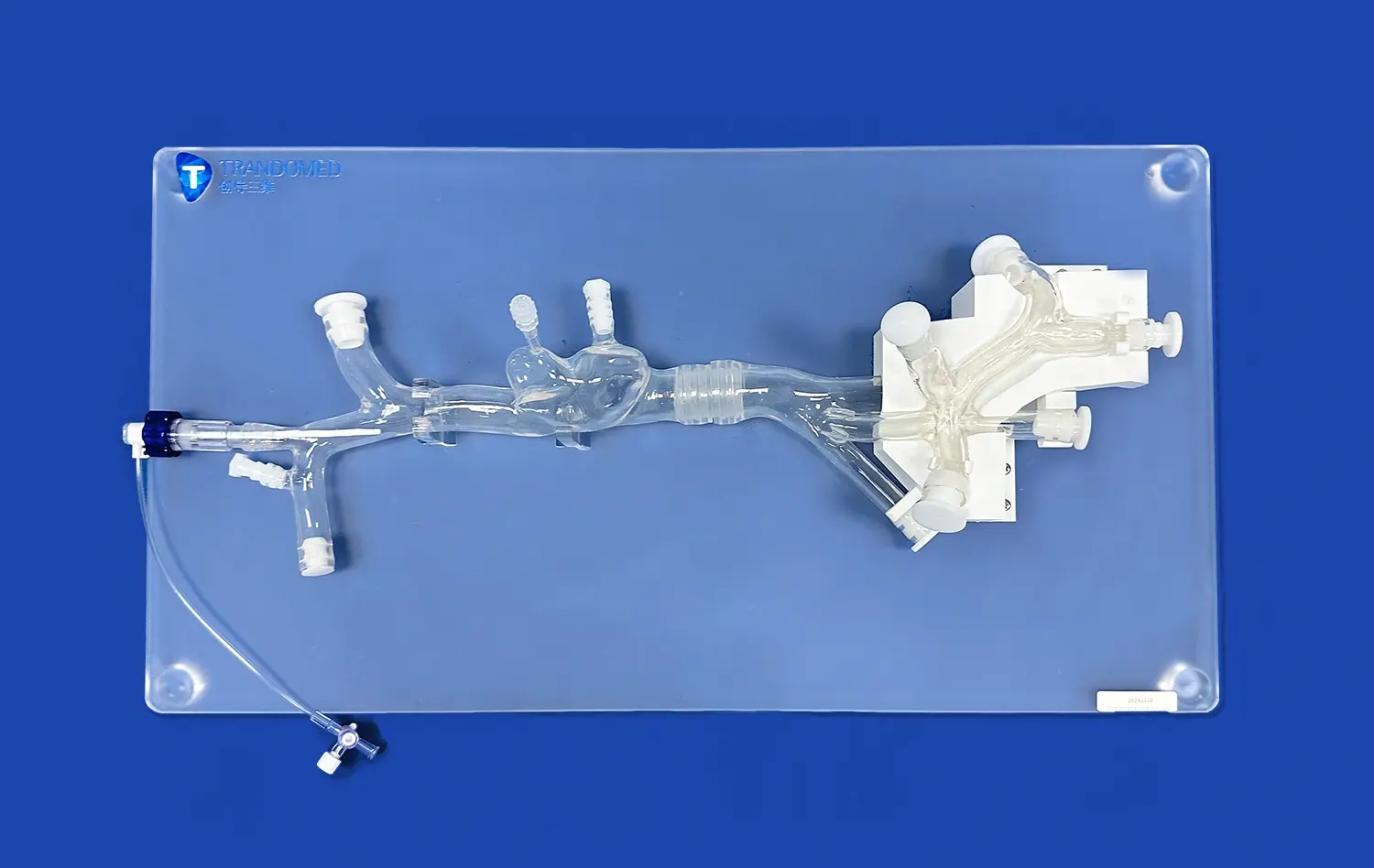
How do Pulmonary Artery Models Ensure Accurate Device Testing?
Anatomical Precision and Physiological Realism
Pulmonary artery models are meticulously crafted to mirror the intricate structures of the human cardiovascular system. Using advanced imaging technologies and 3D printing techniques, these models accurately replicate the dimensions, curvatures, and branching patterns of the pulmonary arteries. This anatomical precision is crucial for evaluating how medical devices navigate through complex vascular pathways, interact with vessel walls, and maintain functionality in tight spaces.Moreover, these models can be designed to simulate various physiological conditions, such as different blood flow rates, pressure gradients, and vessel elasticity. By incorporating materials that mimic the mechanical properties of human tissue, pulmonary artery models provide a realistic environment for assessing device performance under diverse scenarios. This level of physiological realism allows researchers to observe how devices respond to pulsatile flow, vessel compliance, and other dynamic factors that influence their efficacy and safety.
Pathological Variations and Disease States
One of the key advantages of pulmonary artery models is their ability to represent various pathological conditions. Manufacturers can customize these models to include specific anomalies or disease states, such as pulmonary embolism, arterial stenosis, or congenital malformations. This capability is invaluable for testing medical devices designed to treat or diagnose particular conditions.By incorporating these pathological variations, researchers can evaluate how devices perform in challenging clinical scenarios. For instance, a model simulating pulmonary hypertension can help assess the effectiveness of balloon angioplasty catheters or stent deployment systems under increased vascular resistance. This level of specificity in testing enhances the predictive value of validation studies and helps identify potential limitations or complications that may arise in real-world applications.
Reproducibility and Standardization
Pulmonary artery models offer a significant advantage in terms of reproducibility and standardization in device testing. Unlike cadaveric specimens or animal models, which can vary significantly between samples, synthetic models can be produced with consistent specifications. This uniformity allows for more reliable comparisons between different devices or iterations of the same device during the development process.Furthermore, the use of standardized models facilitates collaboration between research institutions and regulatory bodies. When multiple stakeholders use comparable testing platforms, it becomes easier to establish benchmarks for device performance and safety. This standardization not only streamlines the validation process but also contributes to the overall quality and reliability of medical devices reaching the market.
Key Features of Pulmonary Artery Models for Validation Purposes
Multi-level Branching and Vascular Network Complexity
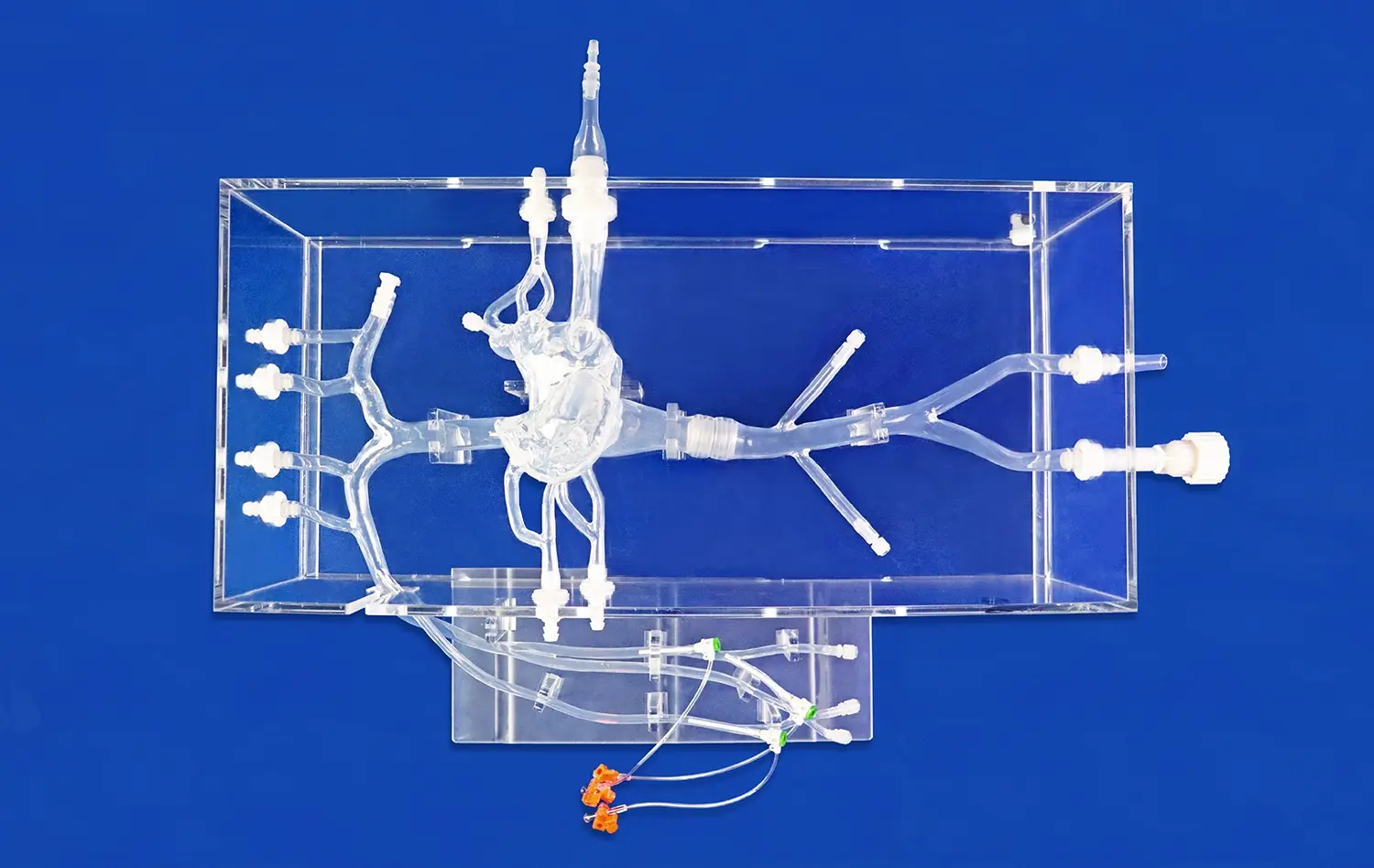
Advanced pulmonary artery models, such as the PA001 from Trandomed, feature intricate multi-level branching systems that accurately represent the complexity of the pulmonary vasculature. These models typically include up to ten levels of bifurcation on both the left and right sides, providing a comprehensive representation of the pulmonary arterial tree. This level of detail is essential for validating devices that need to navigate through progressively smaller vessels or target specific areas within the pulmonary circulation.The complexity of the vascular network in these models allows researchers to assess how devices perform in navigating tortuous paths, crossing bifurcations, and maintaining proper orientation within the vasculature. This feature is particularly important for evaluating guidewires, catheters, and other interventional tools that require precise manipulation through complex anatomical structures.
Customizable Pathologies and Anatomical Variations
One of the most valuable features of modern pulmonary artery models is their customizability. Manufacturers like Trandomed offer the ability to incorporate specific pathologies or anatomical variations into the models. This might include simulations of pulmonary embolism at various locations, arterial malformations, or changes in vessel tortuosity.The customization options extend to adjusting the position and length of simulated embolisms, modifying the intricacy of the inferior vena cava (IVC) section, or altering the overall design of the pulmonary artery to meet specific research requirements. This flexibility allows device manufacturers to test their products under a wide range of clinical scenarios, ensuring that devices are validated for diverse patient populations and pathological conditions.
Material Properties and Tissue Mimicry
The choice of materials used in pulmonary artery models is crucial for accurate device validation. High-quality models utilize materials that closely mimic the mechanical properties of human vascular tissue. For instance, the PA001 model from Trandomed is constructed using silicone with a Shore hardness of 40A, which provides a realistic feel and response to interventional devices.These materials are selected to replicate the elasticity, compliance, and friction characteristics of actual blood vessels. This fidelity in tissue mimicry is essential for assessing how devices interact with vessel walls, evaluating the risk of vascular trauma, and testing the deployment and anchoring of implantable devices such as stents or occluders. The ability to simulate the tactile feedback and mechanical resistance encountered in real procedures enhances the validity of testing results and helps in refining device designs for optimal performance.
Benefits of Realistic Vascular Simulations in Regulatory Approval
Enhanced Safety Profiling and Risk Assessment
Realistic pulmonary artery models play a pivotal role in enhancing the safety profiling of medical devices during the regulatory approval process. By providing a controlled environment that closely mimics in vivo conditions, these models allow for comprehensive risk assessment without endangering human subjects. Manufacturers can thoroughly evaluate potential complications, such as vessel perforation, device migration, or thrombogenic effects, under various simulated scenarios.This meticulous safety evaluation helps identify and mitigate risks early in the development cycle, potentially reducing the need for extensive animal studies and minimizing the likelihood of adverse events during human trials. Regulatory bodies often view data from such detailed simulations favorably, as it demonstrates a proactive approach to safety considerations and a deep understanding of device-tissue interactions.
Accelerated Development and Reduced Time-to-Market
The use of advanced pulmonary artery models can significantly accelerate the development process of medical devices, thereby reducing the time-to-market. These models allow for rapid prototyping and iterative testing, enabling manufacturers to quickly refine designs based on performance data. By identifying and addressing potential issues early in the development cycle, companies can avoid costly setbacks that might otherwise surface during later stages of clinical trials.Moreover, the comprehensive data generated from testing with these models can streamline the regulatory submission process. Well-documented simulation results can provide regulators with a clearer picture of device performance and safety profiles, potentially reducing the number of additional studies required for approval. This efficiency not only saves time and resources but also allows innovative medical solutions to reach patients sooner.
Cost-Effective Validation Strategy
Implementing realistic pulmonary artery models in the validation process offers a cost-effective strategy for medical device manufacturers. While the initial investment in high-quality models may seem significant, it pales in comparison to the potential costs associated with failed clinical trials or post-market recalls due to unforeseen complications.These models allow for extensive testing and refinement without the ethical considerations and logistical challenges associated with animal or human studies. By conducting thorough bench testing with accurate vascular simulations, manufacturers can optimize their designs and protocols before moving to more expensive and complex stages of validation. This approach not only reduces overall development costs but also minimizes financial risks associated with late-stage failures in the approval process.
Conclusion
Pulmonary artery models have emerged as indispensable tools in the validation of medical devices, offering a bridge between theoretical design and clinical application. Their ability to provide anatomically accurate, physiologically relevant, and pathologically diverse testing environments makes them crucial for ensuring the safety and efficacy of cardiovascular interventional devices. By enabling detailed performance assessment, risk evaluation, and iterative refinement, these models not only enhance the quality of medical innovations but also streamline the regulatory approval process. As the field of medical device development continues to advance, the role of sophisticated pulmonary artery models in validation and testing will undoubtedly grow, contributing to better patient outcomes and more efficient healthcare solutions.
Contact Us
Experience the cutting-edge in medical device validation with Trandomed's state-of-the-art pulmonary artery models. Our PA001 model offers unparalleled anatomical accuracy, customizable pathologies, and superior tissue mimicry to ensure your devices meet the highest standards of safety and efficacy. Accelerate your development process, reduce costs, and improve your regulatory approval chances with our advanced simulation solutions. Contact us today at jackson.chen@trandomed.com to learn how our pulmonary artery models can revolutionize your medical device validation process.
1_1732869849284.webp)
_1732843184544.webp)