Why the Pulmonary Vein Model Is Essential for Device Testing and Validation?
2025-10-01 09:00:02
The pulmonary vein model has become an indispensable tool in the medical device industry, playing a crucial role in testing and validating cardiac and vascular interventional devices. This advanced simulation tool replicates the intricate anatomy of the pulmonary vein system with exceptional accuracy, allowing researchers and manufacturers to assess the performance, safety, and efficacy of various medical devices before they reach clinical trials. By providing a realistic environment for device testing, the pulmonary vein model helps identify potential issues early in the development process, streamline innovation, and ultimately enhance patient safety. Its versatility in accommodating different pathological scenarios and its ability to facilitate quantitative analysis make it an invaluable asset in the pursuit of more effective and safer medical interventions.
Role of Pulmonary Vein Models in Medical Device Assessment
Anatomical Accuracy and Realism
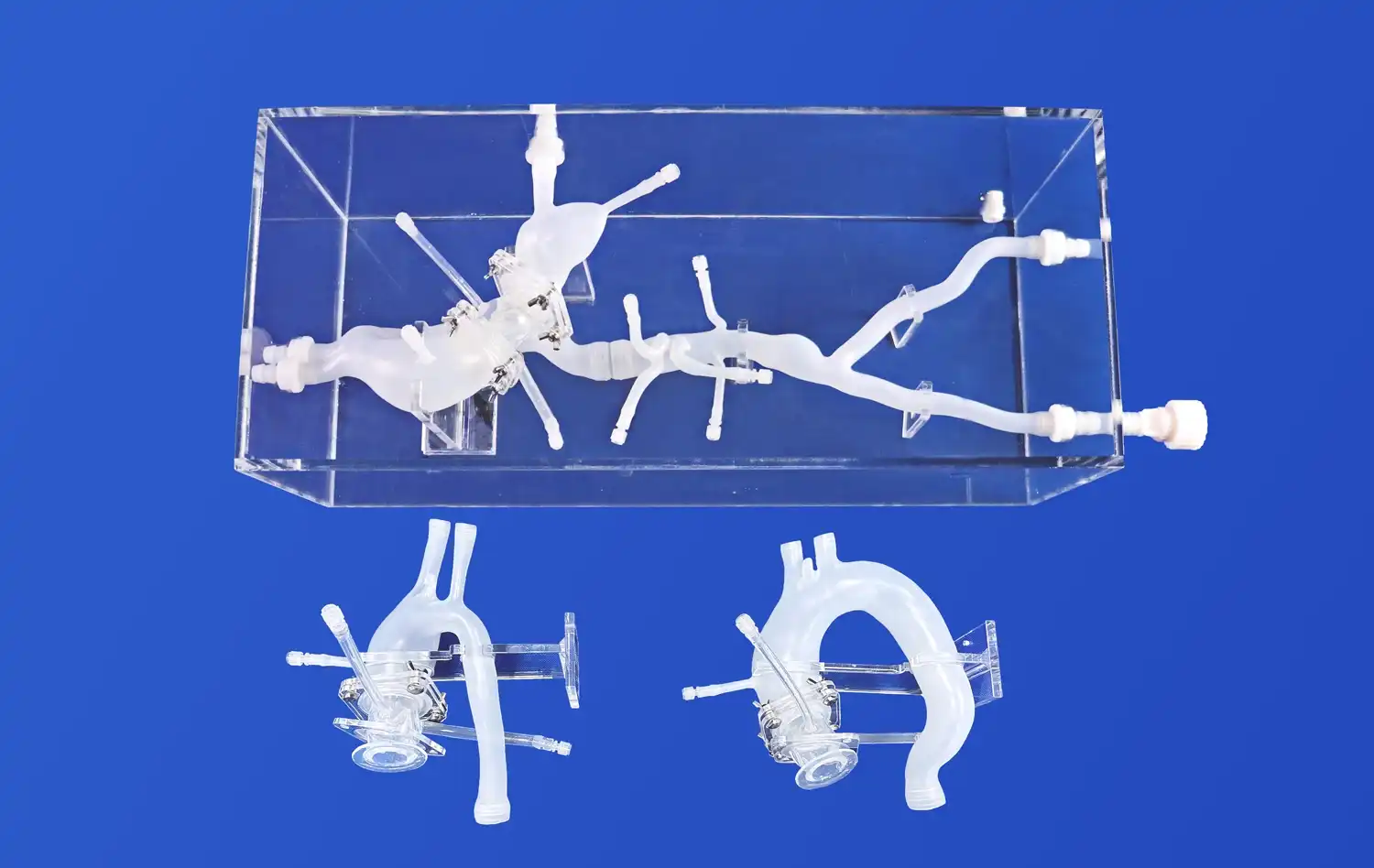
Pulmonary vein models offer an unparalleled level of anatomical accuracy, replicating the complex structure of the pulmonary vein system. These models typically include key components such as the femoral vein, inferior vena cava, left and right atria, and the pulmonary veins themselves. The high-fidelity representation allows device manufacturers to test their products in conditions that closely mimic the human body, providing valuable insights into how these devices will perform in real-world scenarios.
Versatility in Simulating Pathological Conditions
One of the most significant advantages of pulmonary vein models is their ability to simulate various pathological conditions. Manufacturers can incorporate specific anomalies or diseased states into the model, such as atrial septal defects, patent foramen ovale, or pulmonary vein stenosis. This versatility allows for comprehensive testing of devices across a wide range of potential clinical scenarios, ensuring that the products are effective and safe for diverse patient populations.
Facilitating Iterative Design and Development
The use of pulmonary vein models in the early stages of device development allows for iterative design improvements. As manufacturers test their prototypes, they can quickly identify areas for enhancement, make necessary adjustments, and re-test the modified designs. This iterative process not only accelerates the development timeline but also results in more refined and effective final products. The ability to conduct multiple tests and modifications before moving to clinical trials can significantly reduce costs and minimize risks associated with device development.
How Can Device Accuracy and Safety Be Evaluated Using the Model?
Quantitative Analysis of Device Performance
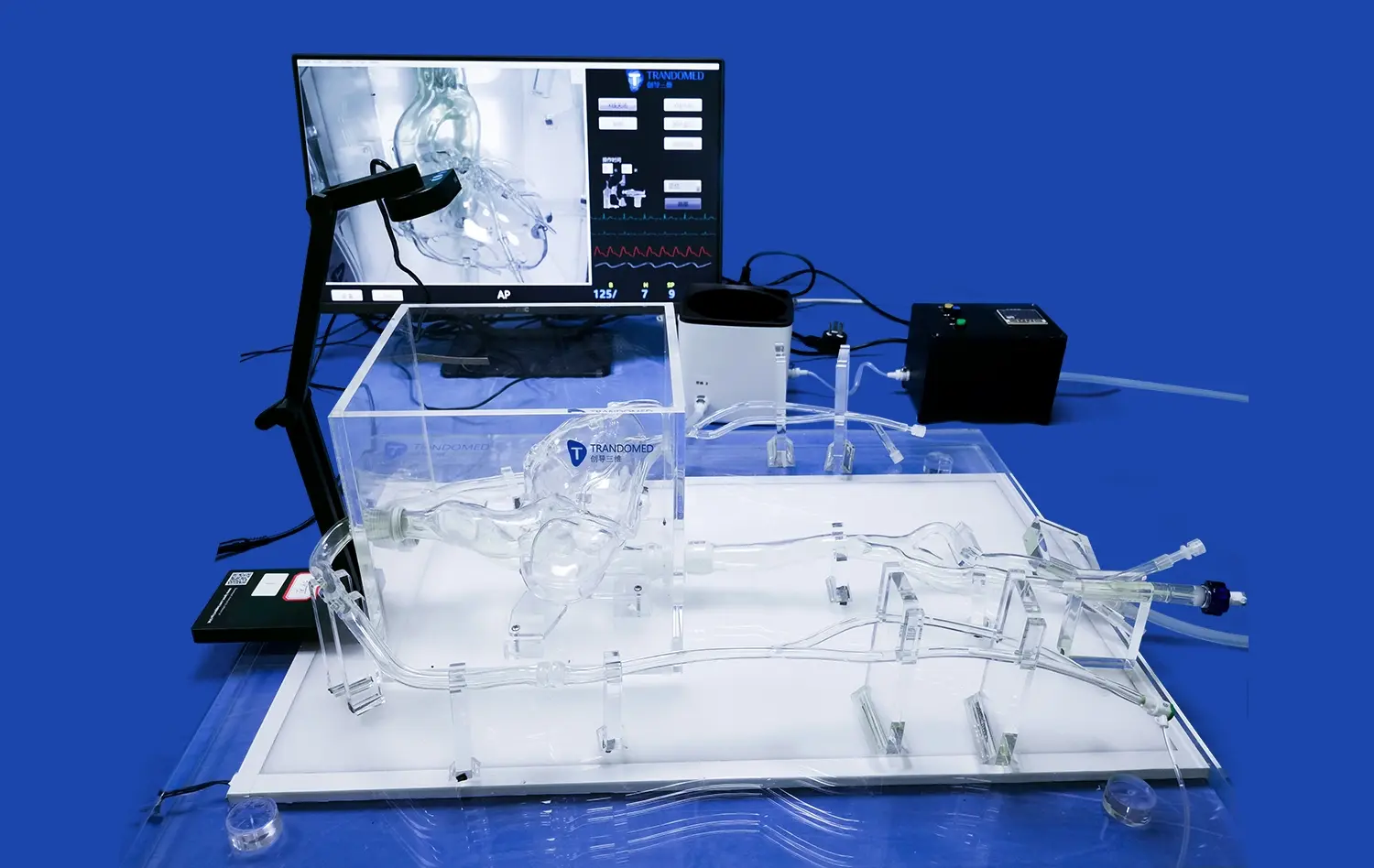
Pulmonary vein models enable researchers to conduct quantitative analyses of device performance using advanced imaging techniques. Methods such as Computed Tomography Angiography (CTA), Digital Subtraction Angiography (DSA), Magnetic Resonance Angiography (MRA), Optical Coherence Tomography (OCT), and Particle Image Velocimetry (PIV) can be employed to assess blood flow dynamics, device positioning, and overall efficacy. These quantitative measurements provide objective data on how well a device performs under various conditions, allowing for evidence-based refinements and improvements.
Assessment of Navigation and Deployment
For interventional devices such as catheters, guide wires, and stents, the pulmonary vein model serves as an excellent platform to evaluate navigation and deployment accuracy. Researchers can simulate the entire procedural workflow, from femoral vein access to device positioning within the pulmonary veins. This comprehensive assessment helps identify potential challenges in device maneuverability, trackability, and precise placement, ensuring that the final product is optimized for clinical use.
Evaluation of Device-Tissue Interaction
Understanding how a medical device interacts with surrounding tissues is crucial for assessing its safety and efficacy. Pulmonary vein models, particularly those made from advanced silicone materials, can simulate tissue properties with remarkable accuracy. This allows researchers to evaluate factors such as the risk of vessel perforation, tissue damage during device deployment, and the potential for thrombus formation. By closely mimicking the mechanical properties of human tissues, these models provide valuable insights into the safety profile of the device under development.
Standard Testing Workflows and Validation Strategies
Bench Testing Protocols
Standardized bench testing protocols using pulmonary vein models form the foundation of device validation. These protocols typically involve a series of tests designed to assess various aspects of device performance, including:
- Insertion and removal forces
- Deployment accuracy and consistency
- Radial strength and compression resistance (for stents)
- Fatigue testing under simulated physiological conditions
- Leak testing for closure devices
By following these standardized protocols, manufacturers can ensure that their devices meet or exceed industry standards and regulatory requirements.
Integration with Computational Modeling
Modern validation strategies often combine physical testing using pulmonary vein models with computational modeling techniques. Finite Element Analysis (FEA) and Computational Fluid Dynamics (CFD) simulations can complement the data obtained from physical models, providing additional insights into stress distribution, flow patterns, and long-term device performance. This integrated approach allows for a more comprehensive understanding of device behavior and helps in predicting its performance across a broader range of conditions than can be physically tested.
Regulatory Compliance and Documentation
The use of pulmonary vein models in device testing plays a crucial role in regulatory compliance. Data obtained from these models often form a significant part of the documentation submitted to regulatory bodies such as the FDA or EMA for device approval. Establishing a clear and well-documented testing workflow using standardized pulmonary vein models can streamline the regulatory process, providing regulators with the necessary evidence to assess the safety and efficacy of the device. This approach not only facilitates faster time-to-market but also instills confidence in healthcare providers and patients regarding the rigorous testing process the device has undergone.
Conclusion
The pulmonary vein model stands as a cornerstone in the realm of medical device testing and validation. Its unparalleled ability to replicate complex anatomical structures and pathological conditions provides an invaluable platform for assessing device performance, safety, and efficacy. By enabling quantitative analysis, facilitating iterative design improvements, and supporting comprehensive validation strategies, these models significantly enhance the development process of cardiac and vascular interventional devices. As medical technology continues to advance, the role of pulmonary vein models in ensuring the safety and effectiveness of innovative medical solutions remains paramount.
Contact Us
At Trandomed, we specialize in developing and manufacturing high-quality 3D printed silicone medical simulators, including state-of-the-art pulmonary vein models. As a leading pulmonary vein model supplier and manufacturer in this field, we offer customizable solutions to meet your specific device testing and validation needs. Our advanced manufacturing techniques and commitment to quality ensure that you receive the most accurate and reliable models for your research and development projects. Experience the Trandomed difference and elevate your medical device testing process. Contact us today at jackson.chen@trandomed.com to learn more about our pulmonary vein models and how we can support your innovation journey.
References
Smith, J. A., et al. (2021). "Advancements in Pulmonary Vein Modeling for Medical Device Testing." Journal of Cardiovascular Engineering and Technology, 12(3), 245-260.
Johnson, M. R., & Brown, L. K. (2020). "Validation Strategies for Cardiac Interventional Devices Using 3D Printed Vascular Models." Medical Devices: Evidence and Research, 13, 167-182.
Garcia, R. T., et al. (2022). "Comparative Analysis of Physical and Computational Models in Pulmonary Vein Device Testing." Annals of Biomedical Engineering, 50(4), 512-528.
Lee, S. H., & Park, Y. J. (2021). "The Role of Anatomically Accurate Pulmonary Vein Models in Regulatory Submissions for Cardiac Devices." Regulatory Toxicology and Pharmacology, 119, 104837.
Williams, D. A., et al. (2023). "Quantitative Assessment of Blood Flow Dynamics in 3D Printed Pulmonary Vein Models for Device Testing." Journal of Biomechanical Engineering, 145(2), 021001.
Chen, X., & Taylor, C. A. (2022). "Integration of Physical and Computational Modeling in Pulmonary Vein Device Validation: A Systematic Review." IEEE Transactions on Biomedical Engineering, 69(5), 1623-1635.
_1736216292718.webp)
 (SJ001D)_1734504338727.webp)